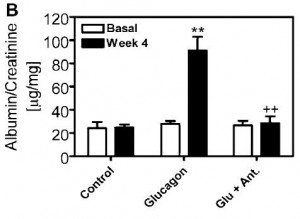
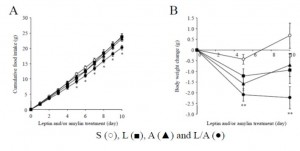
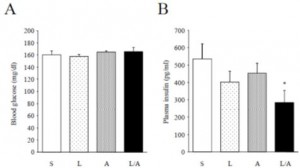
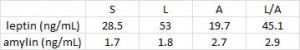
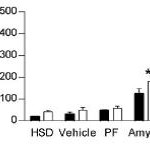
There are many ways to address the etiology of obesity and insulin resistance (or insulin resistance and obesity). For example, you can follow a group of healthy people for a long time and compare those who become insulin resistant with those who don’t; alternatively, you can study a population who is predisposed to insulin resistance (e.g., offspring of type II diabetics)… regarding the latter, although it’s kind of grim, apparently healthy children of obese or diabetic parents are often in an intermediate state of insulin resistance. It’s impossible to exclude a genetic component, but I believe environmental influences are dominant: the poor diet and lifestyle of obese parents is just as likely as obesogenic DNA to be passed on to their children.
The main reason to be concerned with these questions is that there is considerable disagreement about the specific cause of obesity and insulin resistance; i.e., which came first and does one cause the other? Or do they simply share a common cause (e.g., hyperinsulinemia)? I currently lean toward the “common cause” hypothesis. Alternatively, I’d say “it’s complicated” … insulin resistance is not one isolated phenomenon, but the end result of many interconnected biological processes. This has important implications for treatment and prevention- if, for example, hyperinsulinemia causes obesity and/or insulin resistance, then reducing insulin levels or preventing insulin spikes should be prioritized. And mitochondria also seem to be important.
Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes (Morino et al., 2012 Diabetes)
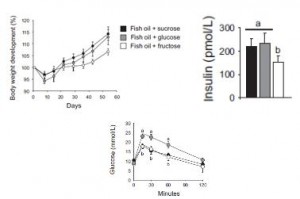
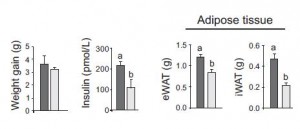
The subjects in this study were body weight and age-matched; the only major difference was impaired glucose tolerance and the presence of at least one diabetic parent in the “insulin-resistant offspring” group. They took muscle biopsies and found, somewhat surprisingly, one of the biggest differences was the content of lipoprotein lipase (LPL).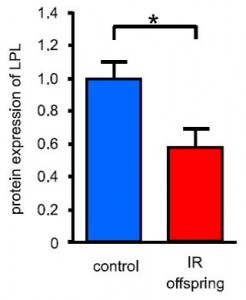 LPL is responsible for hydrolyzing circulating triacylglycerols (from chylomicrons and VLDL) to free fatty acids for tissue uptake. Thus, this finding suggests muscle from insulin-resistant offspring is not as good at sequestering fatty acids (despite these subjects oftentimes having paradoxically higher intramuscular fat levels). This corresponded with lower PPAR activity, mitochondria volume, and fatty acid oxidation. And interestingly, in a set of follow-up cell culture experiments, they found that the fish oil fatty acid EPA (but not DHA) could correct this deficiency.
LPL is responsible for hydrolyzing circulating triacylglycerols (from chylomicrons and VLDL) to free fatty acids for tissue uptake. Thus, this finding suggests muscle from insulin-resistant offspring is not as good at sequestering fatty acids (despite these subjects oftentimes having paradoxically higher intramuscular fat levels). This corresponded with lower PPAR activity, mitochondria volume, and fatty acid oxidation. And interestingly, in a set of follow-up cell culture experiments, they found that the fish oil fatty acid EPA (but not DHA) could correct this deficiency.
Ideally, we would like LPL activated in muscle (to take up and oxidize fatty acids) and inhibited in adipose (to prevent fat cells from getting fatter). Fortunately, there are some relatively easy ways this can be accomplished… exercise selectively activates LPL in muscle and inhibits it in adipose, while insulin does the exact opposite. So eat salmon, exercise, and avoid insulinogenic sugars and carb-rich foods!
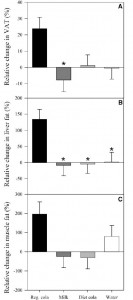
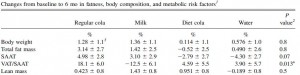
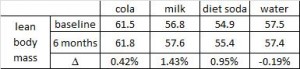
Tissue-specific responses of lipoprotein lipase to dietary macronutrient composition as a predictor of weight gain over 4 years (Ferland et al., 2012 Obesity)
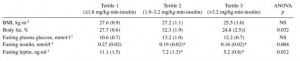
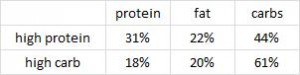
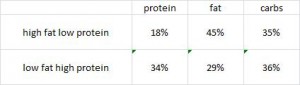
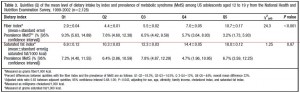
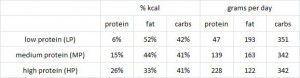
This study was a little more complicated than inferred by the title. First, they took healthy adults, measured body composition and then assessed adipose vs. skeletal muscle LPL activity in the fasted and fed states after 2 weeks of a high fat or high carb diet. To make a long story short: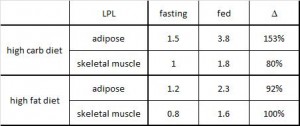 In lean subjects (table above), a high carb meal (after 2 weeks of high carb dieting) markedly increased adipose LPL by 153% (top row) (this is bad), and modestly increased it in skeletal muscle (80%, second row). The high fat meal (after 2 weeks of high fat dieting) caused a smaller increase in adipose LPL (92% vs. 153%) and bigger increase in skeletal muscle LPL (80% vs. 100%) (this is good). Thus, a high carb diet caused the most detrimental changes in adipose LPL while a high fat diet caused the most beneficial changes in skeletal muscle LPL.
In lean subjects (table above), a high carb meal (after 2 weeks of high carb dieting) markedly increased adipose LPL by 153% (top row) (this is bad), and modestly increased it in skeletal muscle (80%, second row). The high fat meal (after 2 weeks of high fat dieting) caused a smaller increase in adipose LPL (92% vs. 153%) and bigger increase in skeletal muscle LPL (80% vs. 100%) (this is good). Thus, a high carb diet caused the most detrimental changes in adipose LPL while a high fat diet caused the most beneficial changes in skeletal muscle LPL.
Next, they compared these acute effects with changes in body composition over the course of 4 years and found that the biggest predictor of increased fat mass was the response of adipose LPL to a high carb diet.
The Morino study showed that increased skeletal muscle LPL was positively associated with insulin sensitivity, while the Ferland study showed that a high carb diet increased adipose LPL and this was positively associated with fat mass gain over 4 years. Skeletal muscle LPL is good, adipose LPL is bad (Rx: EPA [salmon], exercise, and keep insulin levels low).
Dare I say “nutrient partitioning?” this might be one way to reduce body fat without drastically cutting calories. Adopt an LPL-modulating diet and lifestyle! The effect on fat mass not huge, about a pound per year, but that adds up to 10 pounds over the course of a decade… obesity doesn’t happen overnight.
calories proper




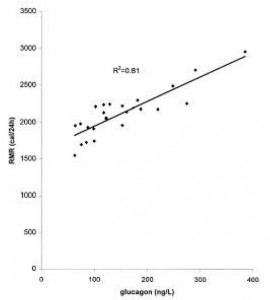
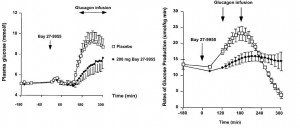
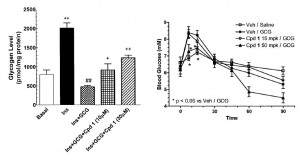
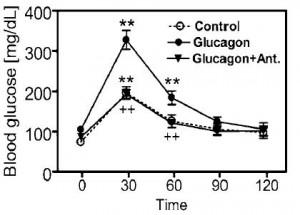









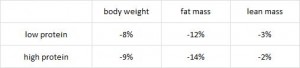


 head over to Patreon
head over to Patreon