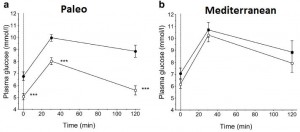
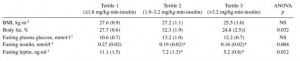
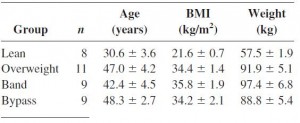
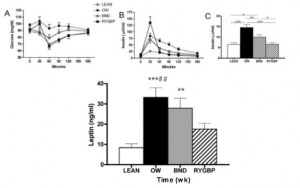
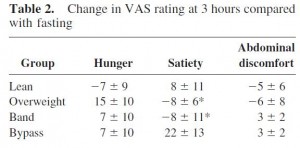
Op. 56
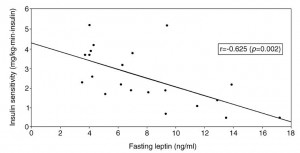
Leptin is probably just as important as insulin WRT obesity, and this is as just as good a place as any to learn about this increasingly interesting hormone.
ABCs of Leptin in a nutshell:
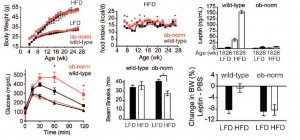
A. Fed state: leptin is secreted from adipose and tells the brain to maintain food intake and energy expenditure at a body weight set point, which is likely established by diet.
B. Fasted state: leptin secretion declines, causing hunger to go up and energy expenditure to go down.
In the past, the cause and consequence of leptin resistance received a lot of my attention due to their importance in obesity. Leptin resistance is, in brief, obesity. Or the mouse on the right:
C. Obesity: eating a poor diet causes leptin resistance, which allows the body weight set point to rise until your fat cells stop responding to insulin (it’s kind of complicated)
But there’s another side of leptin that is mostly unknown, frequently overlooked, and poorly understood. And I say this “is as just as good a place as any” to learn about it because while this side of leptin isn’t as popular as the energy expenditure, appetite, etc., stuff, it could very well be just as important, IMHO.
Leptin vs. the pathological hyperglycemia in diabetic state(s) (note the plural form of “state[s]”).
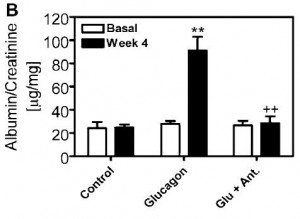
Leptin deficiency causes insulin resistance induced by uncontrolled diabetes (German, Morton, et al., 2011 Diabetes)
Divide and conquer
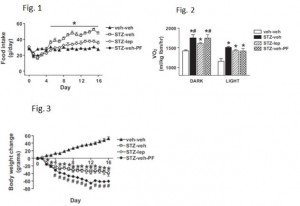
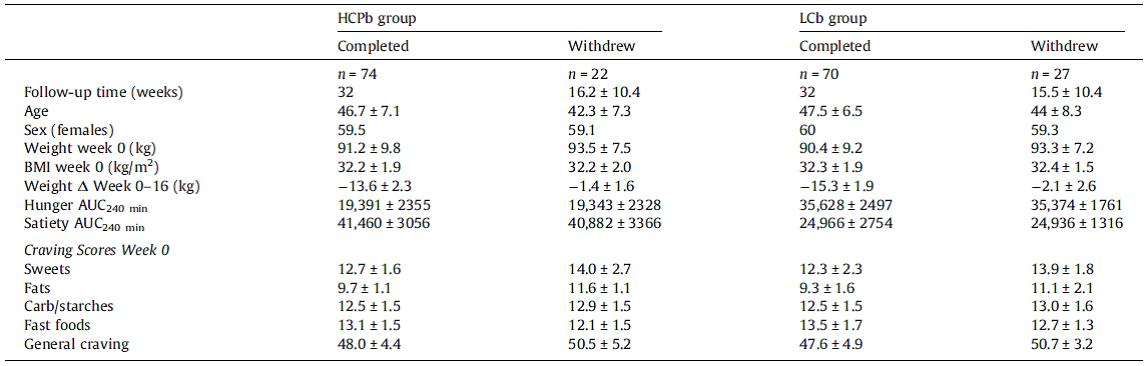
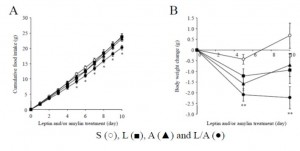
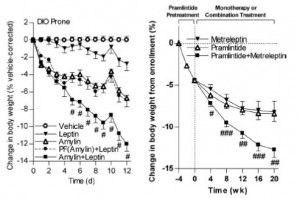
STZ is a beta-cell toxin used to induce diabetes. STZ-treated mice have low insulin, low leptin, and lose weight despite a voracious appetite (just like type 1 diabetic humans). Their insulin resistance is fully corrected while their marked hyperglycemia is attenuated by leptin injections. Leptin reduces food intake but this doesn’t reduce body weight because energy expenditure paradoxically declines (discussed below). Furthermore, diabetic mice restricted to eat only as much as leptin-treated diabetic mice (STZ-veh-PF) lose significantly more weight because they lack the leptin-induced suppression in energy expenditure.
Summary of energy balance:
Control mice (veh-veh) eat the least but have much lower energy expenditure, causing them to weigh the most. Energy expenditure is the more important variable driving high body weight in these animals (it goes down significantly more than food intake). Diabetic mice (STZ-veh) eat the most food which is balanced by high energy expenditure (explained below), causing an intermediate body weight. When the voracious appetite of diabetic mice is restrained (STZ-veh-PF), they weigh the least (they are starving). Food intake is the more important variable driving low body weight in diabetic mice (energy expenditure is the same in diabetic and diabetic-PF mice). STZ-leptin mice have intermediate food intake, energy expenditure, and body weight. All is well, leptin cures the deranged energy balance of type I diabetes.
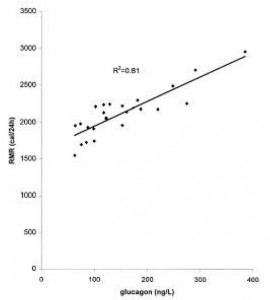
As mentioned above, diabetic mice eat more but weigh less because of drastically increased energy expenditure. Energy expenditure is increased, in part, due to out-of-control gluconeogenesis (from hyperglucagonemia). The paradoxical effects of leptin on energy expenditure (increases it in post-obese subjects but decreases it in diabetic mice) may be explained by leptin-induced reduction of this out-of-control gluconeogenesis, mediated via normalization of glucagon.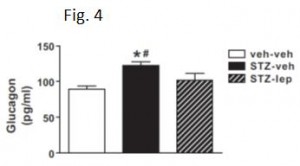
The authors further demonstrated that leptin restores liver, but not muscle or adipose insulin sensitivity in diabetic mice, independent of food intake.
Thus, insulin-deficiency -> dec. leptin -> inc. glucagon -> inc. hepatic glucose output -> hyperglycemia
Insulin’s primarily role might be to suppress glucagon. STZ-induced “relative” state of starvation causes leptin to plummet; in the basal state, leptin may not have anything to do with glucagon because insulin keeps it under control. But in diabetes, there’s no insulin to suppress glucagon; this is where exogenous leptin struts its stuff.
Collectively, these data further support the conclusion that insulin’s major function is to suppress glucagon, as opposed to other effects in skeletal muscle or adipose. It’s been almost a year since Unger’s notorious publication which showed that glucagon receptor knockout mice were immune to type 1 diabetes (discussed here). Diabetic hyperglycemia is partially mediated by insulin resistance, and largely mediated by hyperglucagonemia. But why wasn’t hyperglycemia completely normalized by leptin replacement therapy? These researchers sought simply to normalize leptin levels, but what would’ve happened if they provided a supraphysiological dose of leptin? During physiological leptin replacement therapy, glucagon levels were normalized, but a hint of hyperglycemia remained. Some other [leptin resistant?] member(s) of glucagon’s nefarious cohort must be responsible for the residual diabetic hyperglycemia…
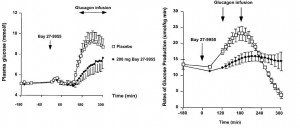
Fortunately for us, the effects of supraphysiological leptin were tested in an identical experimental paradigm:
Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling (Denroche, Kieffer, et al., 2011 Diabetes)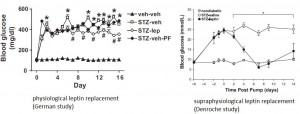
Indeed, supraphysiological leptin therapy overcame whatever diabetic “leptin resistance” remained and totally cured hyperglycemia.
This study repeated much of what was done in the first study, but whereas the first study added that leptin’s effect on food intake was not involved, this study showed that hepatic leptin signaling was not responsible either: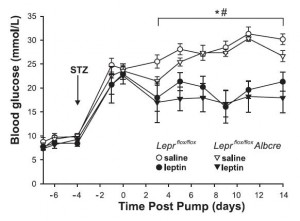
In both cases, hyperglucagonemia appears to be a major cause of diabetic hyperglycemia, and this is cured by leptin. Insulin sensitivity was only partially restored by physiological leptin replacement; this seems to be due to some sort of apparent “leptin resistance,” which is overcome by supraphysiological leptin. Diabetic insulin resistance is most likely caused by hyperglucagonemia-induced increased hepatic glucose output, and this is cured by leptin’s [non-hepatic] effects on reducing glucagon levels. Diabetic insulin resistance may be partially caused by a brain mechanism, but at least one brain mechanism (food intake) was ruled out by the German study.
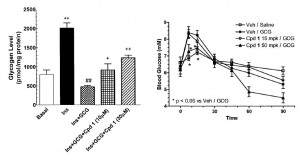
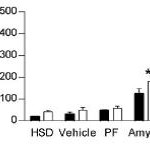
And oh so interestingly, all of these effects were mimicked by leptin administration directly into the brain, at a dose which caused no change in peripheral leptin levels (German, Morton, et al., 2011 Endocrinology). The “STZ-lep” in the figure below refers to diabetic mice with leptin administered directly into the brain.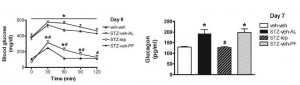
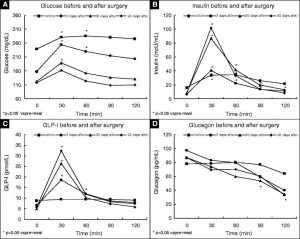
Back to the plural form of diabetic “state[s]” mentioned in the intro. All of the above studies were in insulin-deficient SKINNY type 1 diabetic mice. The next study is in OBESE type 2 diabetic rats.
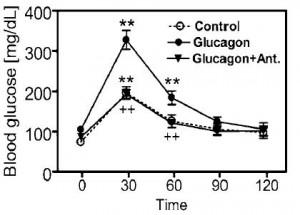
Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats (Cummings, Havel, et al., 2011 PNAS) 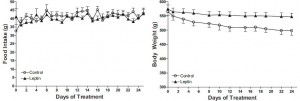
N.B. the control rats in this study were pair-fed to the leptin treated animals to control for the leptin-induced satiation. Leptin-treated mice lost less weight most likely because energy expenditure declined (just like in the first study mentioned above).
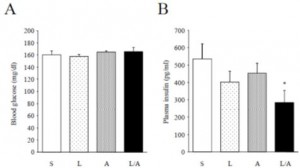
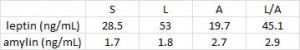
In agreement with the glucose normalization seen by leptin treatment in skinny type 1 diabetic animals, leptin reduced glucose levels in obese type 2 diabetic rats, and this too was associated with reduced glucagon levels: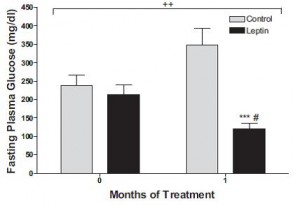
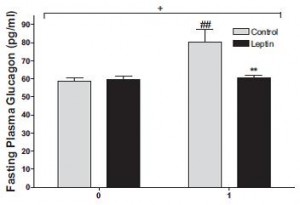 In type 1 diabetic animals, there are very low insulin levels regardless of food intake, leptin treatment, and hepatic leptin signaling. Thus, insulin has nothing to do with the effects of leptin in type 1 diabetes. This study showed that insulin levels also have nothing to do with the effects of leptin in obese type 2 diabetes:
In type 1 diabetic animals, there are very low insulin levels regardless of food intake, leptin treatment, and hepatic leptin signaling. Thus, insulin has nothing to do with the effects of leptin in type 1 diabetes. This study showed that insulin levels also have nothing to do with the effects of leptin in obese type 2 diabetes: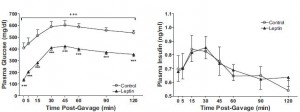
So while the efficacy of exogenous leptin administration in established obesity is questionable, it is capable of combating the pathological glucagon-induced hyperglycemia which is responsible for much of the damage incurred by the diabetic state[s].
Leptin, glucagon, and diabetes.
calories proper
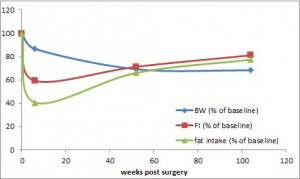
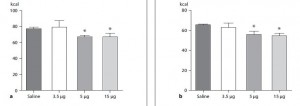
that warrants attention. (it could be the increasing fat intake, but for now let’s just say it’s RYGB per se). According to this pearl, weight loss of only 10% via diet alone causes energy expenditure crash by 394-500 kcal/d, and physiological replacement of leptin via subcutaneous injection can increase this by 234-454 kcal/d (Rosenbaum, Murphy, Heymsfield, Matthews, and Leibel, 2002).