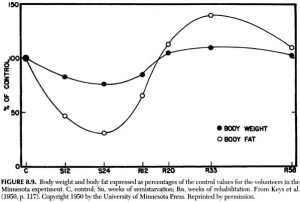
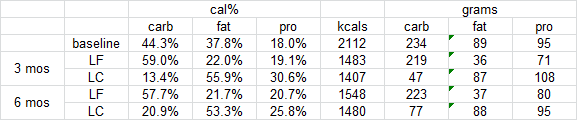
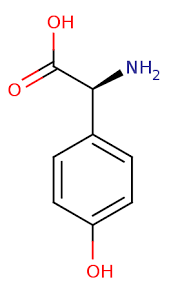
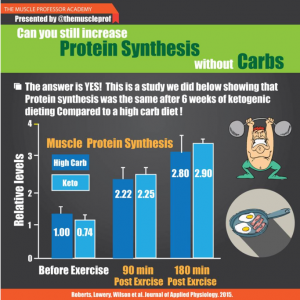
Brief background reading: amylin (according to Wikipedia)
In a study by Hollander on type II diabetics, the synthetic amylin analog pramlintide was tested (Hollander et al., 2003). In this year-long RCT, over 600 patients were treated with placebo or up to 120 ug pramlintide BID (twice per day). On average, these subjects were obese (BMI 34), diabetic for ~12 years, and had an HbA1c of 9.1%. After one year, HbA1c declined 0.62% and they lost about 1.4 kg… not very impressive.
analog pramlintide was tested (Hollander et al., 2003). In this year-long RCT, over 600 patients were treated with placebo or up to 120 ug pramlintide BID (twice per day). On average, these subjects were obese (BMI 34), diabetic for ~12 years, and had an HbA1c of 9.1%. After one year, HbA1c declined 0.62% and they lost about 1.4 kg… not very impressive.
But it’s not all bad news; after viewing those relatively negative results (3 lb weight loss over the course of 1 year), another group of researchers led by Louis Aronne and Christian Weyer believed amylin had yet to be tested proper. So they designed a better study; it was shorter, used higher doses of pramlintide, and they enrolled obese yet non-diabetic patients (Aronne et al., 2007). They opted for higher doses of pramlintide (240 ug TID [three times per day]) because in dose-escalation studies, the incidence and severity of adverse drug reactions was consistently low at all doses tested.
They chose to study obese-er subjects (BMI 38, compared to 34 in the Hollander study) because obese subjects lose fat more readily than lean people, so if the study is designed to measure fat loss, then it is better to select a population of subjects where more fat loss is predicted. They selected non-diabetic subjects for a similar reason; diabetics must regularly inject insulin which promotes the accumulation of fat mass — this could counteract any fat reducing effects of pramlintide.
In other words, it was a more powerful and better designed study.
After 16 weeks, pramlintide-treated subjects lost an average of 3.6 kg (~8 lbs), or about half a pound per week. 30% of patients lost over 15 pounds (1 lb/wk)! Importantly, the weight loss didn’t appear to have reached a plateau by week 16, so it would have most likely continued along a similar trajectory had the study been longer. There were no side effects, and a battery of psychological evaluations showed that the patients receiving pramlintide felt it was easier to control their appetite and BW, they didn’t mind the daily injections, and overall well-being increased. At the very least, these evaluations meant the subjects weren’t losing weight because of nausea or malaise. In fact, it was quite the opposite.
Continue reading →