Trans fats are everywhere. Go to your cupboards, grab any packaged food products like crackers or cereal and you will probably find the words “partially hydrogenated” in the ingredients list. So, what are trans fats? and how bad are they?
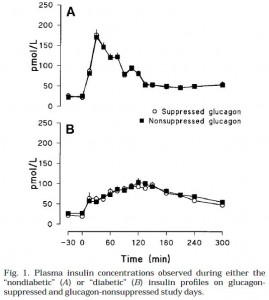
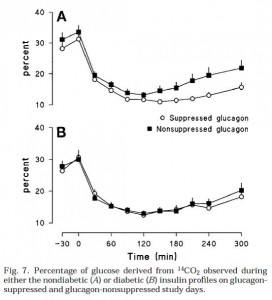
There are two types of fatty acids: saturated (no double bonds) and unsaturated (1 or more double bonds). Fatty acids can be relatively straight molecules, except double bonds in the “cis” configuration put a “kink” in them. “Cis” is the opposite of “trans;” saturated fats are neither cis nor trans because they don’t have any double bonds. See below.
Trans fats are exactly like unsaturated fats except the kink is straightened out a bit, so now they have 1 or more double bond, like unsaturated fats, but are relatively straight, like saturated fats:
It’s kind of amazing that these molecules are so similar, yet you can eat 150 grams of saturated + cis-unsaturated fat every day and live a long healthy life, or eat 5 grams of trans fats, become obese and die prematurely.
Trans fats start out as liquid cis-unsaturated fats in vegetable oils (e.g., soybean oil, canola oil, etc.). From a manufacturing standpoint, cis-unsaturated fats are too soft and unstable. So to put them into foods would result in a messy product that melted at room temperature and then goes rancid. That is not exactly what you think of when you picture a delicious warm muffin, or some crispy crackers, or an oreo cookie. Converting cis-unsaturated fats into trans-unsaturated fats is what makes the difference. Now your favorite processed food products have the perfect consistency, taste delicious, and don’t go stale. From the perspective of the food company, people will buy more because it tastes better, and they don’t have to worry about it going bad if there is a slump in sales. Win-win.
The melting point of the 18-carbon saturated fatty acid “stearic acid” is ~70F (mostly solid at room temperature; e.g., steak fat). The melting point of the 18-carbon monounsaturated fatty acid “oleic acid” is ~56 F (mostly liquid at room temperature; e.g., olive oil). The melting point of the 18-carbon monounsaturated trans-fatty acid “elaidic acid” is 113 F (almost always solid; e.g., Crisco is still solid after sitting out in the Southern California sun all day). FTR, room temperature is 68 – 84F
Stearic acid is pretty much always solid. It is found in steak. Picture the white marbling in a thick cut of beef; it is solid. Unlike oleic acid, which is found in olive oil. Olive oil is usually a liquid, but will solidify if you put it in the fridge for a few hours. Oils high in elaidic acid are almost as solid as stearic acid; elaidic and stearic acids can be solid at body temperature; this is highly favorable for food companies. That is a key temperature that is important for the deliciousness of processed foods; they melt in your mouth not in your hands
Moving on to the data: The first paper is another seminal manuscript from the infamous Nurses’ Health Study. In brief, the Nurses’ Health Study began in the mid-70’s and has been steadily collecting data on over 100,000 women. This particular analysis includes a sample size of ~80,000 (for the record, that is an enormous amount of people to study).
Dietary fat intake and the risk of coronary heart disease in women. (Hu et al., 1997 NEJM)
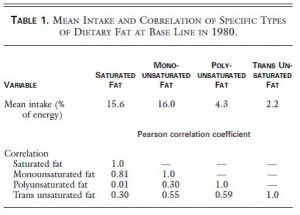
Table 1. top half of the figure, “Mean intake.” On average, in this population of ~80,000 women in the United States, 2.2% of total calories came from trans fat. That’s about 5 grams on a 2,000 kcal diet. Their average total fat intake was 37.1% , or 82 grams per day. Thus, trans fats comprise about 6 % of the total fat ingested.
bottom half of the figure “Correlation.” Trans fat intake correlates better with PUFA & MUFA (0.55 & 0.59) than with SFA (0.30). This is half expected and half surprising. It’s half expected because, as stated above, trans fats are unsaturated fats, so by definition if you’re eating more trans fat then you’re eating more unsaturated fats. It’s half surprising because you might think unhealthy people eat a lot of saturated fat and trans fat, so those two things should correlate well. But they don’t. It appears as though there are some people who eat vegetable oils and trans fats while others who eat saturated fats. There is of course much overlap, but thats the gist of Table 1.
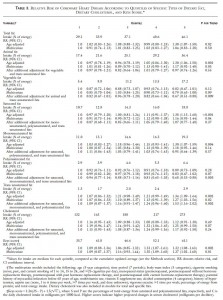
Table 2. What are people eating?

Table 2. I love these kinds of tables; they look busy but really aren’t. There are 4 super-columns: Saturated fat, monounsaturated fat, polyunsaturated fat, and trans unsaturated fat. Each super-column is divided into three sub-columns: low, intermediate, and high intake levels. For example, check out the 1st three columns in the 3rd row: people with low saturated fat intake drink 9 grams of alcohol per day (~1 drink), people with high saturated fat intake drink less (5 grams or about a half a drink).
Now for the fun part: cholesterol (in the red box). Cholesterol and saturated fats are virtually always found in the same foods, so you can see that the people who ate the least saturated fat also ate the least cholesterol (red circle, 183 mg/1000kcal), and people who ate the most saturated fat also ate the most cholesterol (245 mg). Trans fats are primarily made out of vegetable oils, and there is no cholesterol in vegetable oils. People who ate the least trans fats also ate the most cholesterol (218 mg), and people who ate the most trans fat also ate the least cholesterol (206 mg). These tables have the kind of stuff that becomes common sense but only after you think about it for a while.
Back to the half surprising point from above; maybe it should have been only 25% surprising. Healthy people try to avoid both saturated and trans fats, and healthy people who eat the least SFA or trans fat also eat the most fiber (last row in Table 2). So all those things group together and can be seen in the table. The advantage of this kind of table is that it independently breaks down each nutrient. The disadvantage is that sometimes the common sense stuff doesn’t become common sense until you’ve thought about it for a long time, but I guess that’s true with a lot of things.
Moving on, to the bottom half of table 2:

similar trends… People who eat the least saturated or trans fats also exercise the most, smoke the least, etc. Where do you fit in?
Below is Table 3 in its entirety, but don’t worry we will not be considering very much of it.
Divide and conquer.
Of all the variables on that table, Trans fats carry the highest relative risk for CHD: ![]()
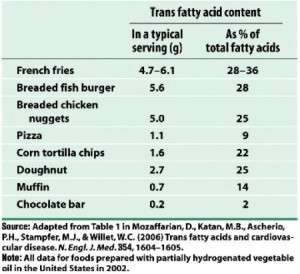
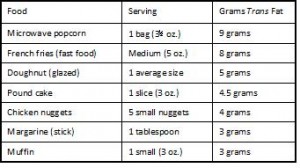
For the highest quintile of trans fat intake compared to the lowest, relative risk is 1.53, or people who consume 2.9% of total calories as trans fats (~8 grams) have a 53% greater chance of developing coronary heart disease than people who consume 1.3% of total calories as trans fats (~2.9 grams). A difference of only 5 grams per day can increase your risk by half! Where can you find 5 grams of trans fat ? I thought you’d never ask:
Holy sh!t 9 grams of trans fat in a bag of microwave popcorn?!? So microwave popcorn causes irreversible lung damage and heart disease too?
(Note the absence of real food on those two charts)
Back to the data: Furthermore, the Nurses’ Health Study seems to exonerate saturated fats a little. The age-adjusted risk for the highest saturated fat intake (18.8% of total calories or 41 grams) compared to the lowest (10.7% or 24 grams per day) is pretty high: 1.38 or 38% greater risk of CHD. Well, back to that ‘common sense’ theme, unhealthy people eat a lot of saturated fat and trans fats. Trans fat is the real bad guy, so when the data are controlled for trans fats, the risk of eating a lot of saturated fat completely went away (see red circle).
The relative risk is “1.07,” which would mean a 7% increased risk for CHD. BUT: The numbers in paranthesis after the relative risk are the confidence interval. In brief, when the confidence interval includes “1.0” as it does here (1.0 is between 0.77 and 1.48), there is no longer a statistically significant association.
And the same thing happened to animal fat:
The adjusted risk for eating 36.4% (81 grams) compared to 17.4% (39 grams) of total calories from animal fat is “0.97” which would mean there is a 3% reduced risk for CHD if you ate more animal fats. BUT the confidence interval includes “1” so there is no statistically significant association.
In the Nurses’ Health Study of ~80,000 women in the United States, CHD risk was not associated with intake of saturated fat, animal fat (or cholesterol), and was significantly associated with trans fat. My take? If you are at risk for CHD, avoid trans fats like the plague. And microwave popcorn.
calories proper