…just read that huge disasters, ranging from Exxon Valdez to Chernobyl, may have been due, in part, to ignorance of basic principles of circadian rhythms. Gravitas.
…just read that huge disasters, ranging from Exxon Valdez to Chernobyl, may have been due, in part, to ignorance of basic principles of circadian rhythms. Gravitas.
Posted in Advanced nutrition, chronopharmacology, circadian, diet, melatonin, mortality, pair-feeding, Protein, sleep, Sun
Tagged circadian rhythm, diabetes, melatonin, mortality, nutrition, protein
“Desynchronization between the central and peripheral clocks by, for instance, altered timing of food intake, can lead to uncoupling of peripheral clocks from the central pacemaker and is, in humans, related to the development of metabolic disorders, including obesity and type 2 diabetes.”
If you haven’t been following along, a few papers came out recently which dissect this aspect of circadian rhythms — setting the central vs. peripheral clocks.
In brief (1): Central rhythms are set, in part, by a “light-entrainable oscillator (LEO),” located in the brain. In this case, the zeitgeber is LIGHT.
Peripheral rhythms are controlled both by the brain, and the “food-entrainable oscillator (FEO),” which is reflected in just about every tissue in the body – and is differentially regulated in most tissues. In this case, the zeitgeber is FOOD.
In brief (2): Bright light in the morning starts the LEO, and one readout is “dim-light melatonin onset (DLMO),” or melatonin secretion in the evening. Note the importance of timing (bright light *in the morning*) – if bright light occurs later in the day, DLMO is blunted: no bueno.
Morning bright light and breakfast (FEO) kickstart peripheral circadian rhythms, and one readout is diurnal regulation of known circadian genes in the periphery. This happens differently (almost predictably) in different tissues: liver, a tissue which is highly involved in the processing of food, is rapidly entrained by food intake, whereas lung is slower.
Starting the central pacemarker with bright light in the morning but skimping on the peripheral pacemaker by skipping breakfast represents a circadian mismatch: Afternoon Diabetes? Central and peripheral circadian rhythms work together. Bright light and breakfast in the morning.
Posted in Advanced nutrition, chronopharmacology, circadian, liver, melatonin, Protein, sleep, Sun
Tagged circadian rhythm, melatonin, nutrition, obesity, protein
“Without leaves, without buds, without flowers;
Yet they form fruit.
As a Food, as a tonic, as a medicine;
The entire creation is precious.”
-weird mushroom poem of sketchy origin
Mushrooms: They have B12! When exposed to UV light, they make vitamin D2. Protein, fibre, and selenium. Shall I go on?
Posted in Advanced nutrition, microbiota, Protein, Sun, Vitamin D
Tagged calories proper, carbs, mushrooms, nutrition, prebiotics
“Wait… what? nutrient partitioning?”
Calories In, Calories Out should not be interpreted as “eat less, move more,” but rather kept in its more meaningless form of: “if you eat less than you expend, you’ll lose weight.” At least then, it’s correct… meaningless, but correct. Eating less and moving more is no guarantee of fat loss, in part, because total energy expenditure isn’t constant and there’s that whole thing with nutrient partitioning.
For obese insulin resistant folks, this is Low Carb’s strong suit: it causes “eat less, move more”spontaneously.
For some obese insulin sensitive patients, for whatever reason, their adherence and success is greater with Low Fat. You might say, “yeah, but those suckers had to count calories.” To that, I’d counter with: “it doesn’t matter, THEY WERE MORE SUCCESSFUL COUNTING CALORIES ON LOW FAT THAN NOT COUNTING ON LOW CARB.” The spontaneous reduction in appetite obviously didn’t cut it. Do not be in denial of these cases.
Posted in Advanced nutrition, Dietary fat, Energy balance, Grains, insulin, Protein
Tagged body composition, calories, carbs, diet, energy balance, energy expenditure, fat, grains, nutrition, obesity, Paleo, protein
I didn’t want to blog about the artificial sweetener study; to be honest, I didn’t even want to read it. I just wanted to report: 1) how many Diet Cokes are we talking about; and 2) when are you going to die.
Artificial sweeteners induce glucose intolerance by altering the gut microbiota (Suez et al., 2014)
Non-caloric artificial sweeteners (NAS) = saccharin, sucralose, and aspartame. Saccharin worked the best (worst) in the mouse study, so they tested it in humans. This was the part I found most relevant: seven healthy volunteers (5 men & 2 women, aged 28-36) who did not typically consume a lot of sweeteners were recruited and given 120 mg saccharin three times per day. 360 mg saccharin is ~10 packets of Sweet’n Low.
Posted in Advanced nutrition, fermentation, microbe, microbiome, microbiota, Protein, Sugar
Tagged carbs, diet, microbiota
Don’t exacerbate afternoon diabetes with afternoon carbs.
Skeletal Muscle
As discussed previously [at length], insulin sensitivity in skeletal muscle follows a circadian pattern: starts out high in the morning and wanes throughout the day.
Diurnal variation in glucose tolerance and insulin secretion in man (Carroll and Nestel, 1973)
Adipose Tissue
And insulin sensitivity of adipose tissue goes in the opposite direction: starts out low, and increases as the day progresses.
Diurnal variations in peripheral insulin resistance and plasma NEFA: a possible link? (Morgan et al., 1999)
The studies were standardized for a period of fasting, pre-test meal, and exercise… Following insulin, NEFA fell more slowly in the morning (149 uM/15 min) than in the evening (491 uM/15 min).
Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids (Zimmet et al., 1974)
Adipose tissue insulin sensitivity is greater in the evening. FFA are higher, and get shut down more rapidly, after a carb meal in the evening.
Summary: to minimize blood glucose excursions and proclivity for fat storage, eat more calories earlier in the day; this is circadian nutrient timing. And according to the Alves study, a low-carb protein-rich dinner best preserves lean tissue during weight loss.
Posted in Advanced nutrition, circadian, clamp, diet, Energy balance, insulin, Ketosis, melatonin, Protein
Tagged body composition, calories proper, carbs, circadian rhythm, energy expenditure, insulin, ketosis, melatonin, nutrition, protein
This is where the magic happens.
Rat pups, fed a flaxseed oil-based ketogenic diet from weaning onward – note the drop-off in ketones after 2 weeks (Likhodii et al., 2002):
What happened on day 17?
Patient history: these rats have been “low carb” their whole lives.
Side note: flaxseed oil is very ketogenic! (Likhodii et al., 2000):
Flaxseed oil-based ketogenic diet produced higher ketones than 48h fasting; the same can’t be said for butter or lard. PUFAs in general are more ketogenic than saturated fats in humans, too (eg, Fuehrlein et al., 2004):
Crisco keto (adult rats) (Rho et al., 1999):
suspect those two rogue peaks were experiment days…
Posted in Advanced nutrition, circadian, diet, Dietary fat, Exercise, insulin, Ketosis, Protein
Tagged Atkins, body composition, carbs, energy expenditure, fat, insulin, ketogenic, ketosis, protein
*ugh* journalists
I’m talking to you, Mandy Oaklander!
Regarding the new low carb vs low fat study, she writes: “Popular diets are pretty much the same for weight loss, study finds.”
Effects of low-carbohydrate and low-fat diets: a randomized control trial (Bazzano et al., 2014)
Further, “An earlier study in Annals of Internal Medicine did find that low-carb dieters lost slightly more weight than low-fat dieters after one year. The study today reached similar conclusions, but the differences in weight loss were not significant.”
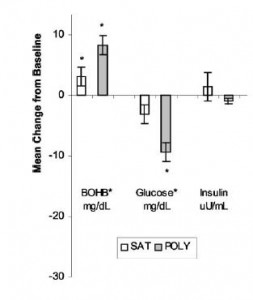
Perhaps Mandy just doesn’t realize there’s a difference between significant, as in “meaningful,” and significant, as in “P<0.05.” Pro-tip: you can tell them apart relatively easily, because the latter is usually accompanied by a cute little asterisk. For example, the differences in weight loss were quite statistically significant (P<0.05):
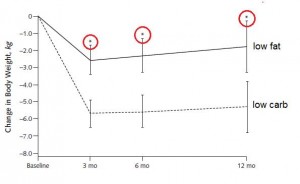
She goes on to say “After a year follow-up, some of those pounds crept back for people on both diets…”
To that I say: yeah, but fat mass continued to decline in those on the low carb diet, meaning some of that weight re-gain was muscle:
So, between 6 and 12 months, carbs and calories were creeping up in the LC group, yet fat mass was still declining. Perhaps this way of eating improved their metabolism, or restored the ability to effectively partition nutrients.
***in real-time: at this point, I realize that Mandy was actually talking about the other study, which she was covering accurately. Sorry, Mandy!***
…so maybe the low-carb (LC) diet improved muscle mass because it was also high protein
? …perhaps, but 19% vs 24% (71 vs 85 grams) isn’t a very big difference. Alternatively, since the LC group really just maintained absolute protein intake (86 grams at baseline, 85 at month 12), whereas low-fat (LF) dieters decreased (86 grams at baseline, 71 at 12 months); perhaps this is why LF lost muscle mass..? Still, those changes in protein intake are small, and I think people can be too quick to chalk up the benefits of LC to “high protein.”
In sum, this is actually one of the more “pro” LC studies. And it wasn’t even a huge difference in carbs: 198 vs 127 grams/d at month 12 (54% vs 34%). Big difference in fat mass; and CRP, a marker of inflammation, even declined in the LC group.
Low fat diet advocates have been giving me headaches for years… the low fat diet caused headaches (P<0.05):
The study Mandy was actually talking about: Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis (Johnston et al., 2014)
It was a meta-analysis, which is just about the only type of study capable of taking down LC.
@JohnPiersonPT @trendclues @CaloriesProper @samiinkinen @BradSchoenfeld Ah yes, meta-analysis. pic.twitter.com/MSnK5L1eb4
— Gerard Pinzone (@GerardPinzone) September 3, 2014
…but at least it had this cool chart (modified):
*ugh* scientists
The macro’s in “Low fat” overlap with “Moderate,” implying “Low carb” is “EXTREME” …the authors’ bias is subtle, I’ll give ‘em that, but I’m getting too old for this.
Dear Obesity Researchers,
If you want to design a study showing a low fat diet is as good as low carb for fat loss, here’s your best bet: recruit young, exercise-tolerant overweight patients who aren’t on any meds. PROOF (see Ebbeling study). Or find 10 similar ones and write up a pro-LF meta.
If you want to show low carb is better, recruit patients with obesity.
Posted in Advanced nutrition, diet, Dietary fat, empty calories, Energy balance, Exercise, fat, insulin, Protein
Tagged Atkins, body composition, calories proper, carbs, diet, energy balance, fat, insulin, obesity, protein
Meal & exercise timing in the contexts of “damage control” and nutrient partitioning are frequent topics on this blog. I generally opt for a pre-workout meal, but nutrient timing hasn’t panned out very well in the literature. That’s probably why I’m open to the idea of resistance exercise in the fasted state. A lot of pseudoscientific arguments can be made for both fed and fasted exercise, and since a few blog posts have already been dedicated to the former, this one will focus on the latter.
The pseudoscience explanation is something like this: since fatty acids are elevated when fasting, exercise in this condition will burn more fat; and chronically doing so will increase mitochondria #. The lack of dietary carbs might enhance exercise-induced glycogen depletion, which itself would bias more post-workout calories toward glycogen synthesis / supercompensation. Much of this is actually true, but has really only been validated for endurance training (eg, Stannard 2010, Van Proeyen 2011, & Trabelsi 2012; but not here Paoli 2011)… and the few times it’s been studied in the context of resistance exercise, no effect (eg, Moore 2007 & Trabelsi 2013). However, there are some pretty interesting tidbits (beyond the pseudoscience) which suggest how/why it might work, in the right context.
John Kiefer, an advocate of resistance exercise in the fasted state, mentioned: “the sympathetic nervous system responds quicker to fasted-exercise. You release adrenaline faster. Your body is more sensitive particularly to the fat burning properties of adrenaline and you get bigger rushes of adrenaline.”
Much of this is spot on. That is, ketogenic dieting and glycogen depletion increase exercise-induced sympathetic activation and fat oxidation (eg, Jansson 1982, Langfort 1996, & Weltan 1998).
The question is: can this improve nutrient partitioning and physical performance? Magic 8-Ball says: “Signs point to yes.” I concur.
Contrary to popular beliefs, glycogen depletion per se doesn’t harm many aspects of physical performance. A lot of fuel systems are at play; you don’t need a full tank of glycogen.
Increased fat oxidation compensates for reduced glycogen at lower exercise intensities (eg, Zderic 2004), and ketoadaptation may do the same at higher intensities.
Posted in Advanced nutrition, circadian, diet, endurance, Energy balance, Exercise, insulin, Ketosis, muscle, Protein, TPMC
Tagged body composition, calories proper, carbs, diet, energy balance, energy expenditure, exercise, insulin, ketosis, muscle, nutrition, protein
One way to determine protein requirements is the nitrogen balance technique. If all of the nitrogen from dietary protein intake is equivalent to that lost via feces, urine, and sweat, then one is in nitrogen balance. Growing children and pregnant women are usually in positive nitrogen balance, because much of the nitrogen is being invested in the growth of new tissue. Cachectic cancer patients and sarcopenic elderly may be in negative nitrogen balance, because they’re losing lean mass.
Protein requirements to maintain nitrogen balance are largely dependent on total energy intake. More calories in, less protein needed. For people in negative energy balance (losing weight), this usually means more protein is required else muscle will be wasted.
Exercise lowers, not raises, protein “requirements,” because exercise is a potent anabolic stimulus; it helps preserve nitrogen at any level of dietary protein. That’s not to say more won’t improve functional outcomes; just that it’s not “necessary” to prevent muscle loss.
Need =/= optimization.
Lastly, total grams, not percent of calories, is the most relevant way to talk about protein requirements in the context of nutrient partitioning and body composition. This is just how protein operates.
Part 2. The poor, misunderstood Randle Cycle
“The glucose-sparing effect of fat-derived fuels” …when you’re body starts burning more fat (and fat-derived fuels; ie, ketones), it’s use of glucose declines. Thus, it’s “glucose-sparing” (spares glucose for the brain and obligatory glycolytic tissues, yada yada yada).
During starvation, much of that glucose comes from amino acids from skeletal muscle proteins, so it can also be phrased as: “the muscle-sparing effect of fat-derived fuels,” which is equally biologically relevant, because similar to zeroglycemia, an unabated loss of muscle is incompatible with survival.
That is, in starvation, where the “protein” is skeletal muscle, not dietary (because starvation)… but what about when following a low carb or ketogenic diet – do ketones (fat-derived fuels) exert a muscle-sparing effect in this context?
One study compared the impact of two isonitrogenous diets, low carb (Diet A) vs. high carb (Diet B), on nitrogen balance and showed that, except at very high levels of energy intake, nitrogen balance was consistently better on high carb.
However, 51 kcal/kg is the textbook number of kcals “required” for young, moderately active adults. With this understanding, it could be interpreted to mean that nitrogen balance is better with low carb (Diet A) for people in energy balance; and better with high carb (Diet B) if energy deficit.
edit: 51 kcal/kg is for athletes; probably about 20-25% less for non-athletes.
Or not: in another study, a low carb diet promoted better nitrogen retention albeit less weight loss than an isocaloric low fat diet. The low carb group lost slightly more fat mass, which, combined with nitrogen balance data, suggest modestly improved body composition. The differences were small, because this was a “non-ad lib” isocaloric diet study. In the absence of large differences in intake, the most we can expect from such studies are subtle alterations in nutrient partitioning (which are usually difficult to detect).
Cancer cachexia is a condition of severe muscle wasting, and one study set out to determine, more directly, if ketones spared muscle in this context. The study only lasted one week, but I suspect a certain degree of expedited ketoadaptation because: 1) it was very low in carbohydrate; 2) the fat was primarily MCTs; 3) they supplemented oral ketones; and 4) energy expenditure is elevated in this population. Both the control and ketogenic diets were modestly hypercaloric, but nitrogen balance was more favorably improved by the high carb diet, in contrast to the above studies.
Thus, ketones don’t work in the context of a hypercaloric diet; however, pharmacologically elevating ketones via intravenous infusion in fasting subjects does work (because it’s more like starvation).
The muscle-sparing effect of fat-derived fuels is conceptually and physiologically more relevant to starvation, not nutritional ketosis.
Part 3. Protein “requirements”
Protein intake was 1x, 2x, or 3x the RDA; fat was 30% of calories, and carbs made up the rest; on a weight maintenance diet and again on 30% calorie restriction (it was technically a 40% energy deficit, because they tried to ramp up energy expenditure with exercise).
All groups lost weight, but the ratio of fat to muscle loss was significantly higher in the 2x and 3x RDA groups, which amounted to ~120 and 185 grams of protein per day, respectively. The 3x group didn’t fare as well, possibly, because that much protein induces a high degree of satiety – this group ended up consuming significantly fewer calories than the 2x group. So the interplay between energy intake and protein requirements is back on the table: the added energy deficit apparently increased protein requirements to some level above 185 grams per day. Not much, given the small difference in muscle loss, but increased none the less.
Side note: be cautious when interpreting a study about the amount of protein required for xyz endpoint, because such studies usually only measure one of many important markers, and they don’t report absolute changes in size, strength, etc. Also, context matters.
For example, Moore and colleagues (2014) showed that 0.24 g/kg (17 grams for a 70 kg adult) was enough to maximally stimulate myofibrillar fractional synthetic rate (mFSR):
However, in the contexts of three square meals and energy balance (or deficit), 0.72 g/kg (50 g/d) is woefully inadequate. Point being: mFSR (in this case) is only one measurement and shouldn’t be extrapolated to total daily requirements. Perhaps you could eat six 17 g servings in order to fully maximize 24-hour mFSR, or you could realize that going above what saturates mFSR isn’t a bad thing, or wasteful. mFSR is just one of many measurements of muscle protein balance.
My opinion
For those who need exact numbers, hopefully one point I’ve made is that there’s no answer to this question. I’d guess that most people “need” 100+ grams of protein per day (more if losing weight), and 100 grams is probably too much in one sitting. Also, need =/= optimization, and context matters.
Nutritional ketosis doesn’t appear to reduce the amount of dietary protein necessary to maintain lean mass. The muscle-sparing of fat-derived fuels works during starvation; in other contexts, all bets are off.
Posted in Advanced nutrition, diet, Dietary fat, Energy balance, fat, insulin, Protein
Tagged body composition, calories, calories proper, carbs, diet, empty calories, energy balance, energy expenditure, fat, insulin, nutrition, protein