Insulin is there to grow fat tissue for the obesity epidemic, not replenish glycogen after yoga.
Teaser: insulin-induce hypoglycemia can get deadly quite fast, and there is no equivalent for the effects of insulin on fat. However, the effects of insulin on fat are 100 times more powerful.
Background: Hormone sensitive lipase (HSL) responds to insulin by inhibiting lipolysis. It halts fat burning. It got its name because it’s THEE most hormone-sensitive lipase in the body. The hormone about which we are speaking is of course insulin. And the enzyme, or at least one of the enzymes as it were, is HSL. To be clear, it takes very little insulin to inhibit HSL. Just a dollop, in fact.
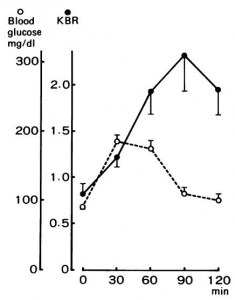
Effect of very small concentrations of insulin on forearm metabolism. Persistence of its action on potassium and free fatty acids without its effect on glucose. (Zierler and Rabinowitz, 1964)
Expt 1. Since we’re all about jabbing people with insulin lately, let’s get at it again. Jab someone with about 100 uU (/min*kg), and muscle and fat vacuum glucose out of the blood. Same goes for potassium; and adipose gets all stingy too… it stops releasing and starts storing fat. This is “healthy,” and its part of why people say insulin, and by extension carbohydrate, causes lean people grow fat tissue.
What do you think would happen in an insulin resistant obese crowd. Less glucose vacuuming, but scrooge adipose will still responds with gravitas, by saving more and spending less? Likely. HSL is like the little piggy’s straw house. The strong young wolf can blow it down. The COPD emphysema wolf can blow it down… because it’s made of straw.
Thus, insulin causes lean people to grow fat tissue, and it causes obese people to grow more fat tissue.
In other words, with regard to common obesity, being resistant to insulin means postprandial hyperglycemia; you can’t handle sugars proper. but it’ll still make you fat(ter).
Expt 2. The interesting part. Try jabbing healthy people with 10x less insulin. Looks like IR obesity! Adipose gets stingy, potassium scrams, but no effect on glucose uptake.
In the figure below: A-DV is muscle; A-SV is adipose. Glucose uptake into fat & muscle is unaffected by a low dose of insulin.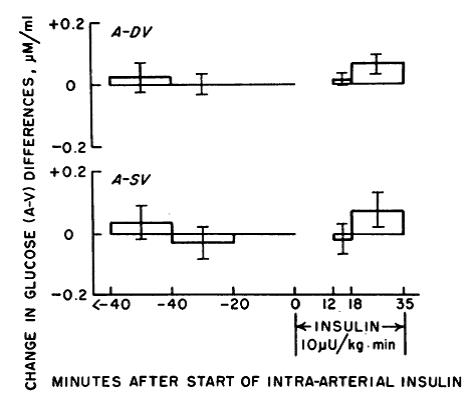
Second figure: with the same dose, adipose goes on a budget SAVE MORE SPEND LESS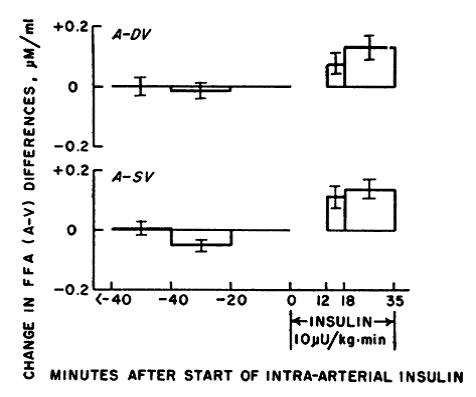
Conclusion. In a healthy person, (eg, 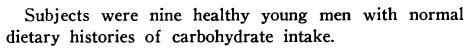 ), even very low doses of insulin cause fat growth. This isn’t an issue of high vs. low glycemic issue. The insulin dose used in this study was less than that expected from a respectable low glycemic index meal. This is probably why the glycemic index hasn’t cured the obesity epidemic. On the other hand, dietary fat doesn’t stimulate insulin… just sayin’
), even very low doses of insulin cause fat growth. This isn’t an issue of high vs. low glycemic issue. The insulin dose used in this study was less than that expected from a respectable low glycemic index meal. This is probably why the glycemic index hasn’t cured the obesity epidemic. On the other hand, dietary fat doesn’t stimulate insulin… just sayin’
Furthermore, perhaps glucose uptake into adipose promotes fat storage under certain conditions, but it’s clearly neither necessary nor essential. Insulin can Miracle Grow fat mass without affecting glucose uptake one iota. I imagine the abundance of 3C precursors simply isn’t “the limiting factor.” And it works just as good with Whole Foods Low GI pa$ta and Wonderbread.
Translation: insulin buttresses fat growth. and it doesn’t matter how much. FYI this probably seems nonsensical at first: carbs stimulate insulin in order to dispose of said carbs, like a logical feedback mechanism. Perhaps. But said insulin cares far more about fat than said carbs. On a scale of 1 to 10 (ie, putting things into “perspective”): insulin is there to grow fat tissue for the obesity epidemic, not replenish glycogen after yoga.
Part II.
Dose-dependent effect of insulin on plasma free fatty acid turnover and oxidation in humans (Bonadonna et al., 1990)
There are a lot of data in this paper, but here are the relevant points:
Infuse insulin at various rates. In the lowest infusion rate, the only aspect of glucose metabolism to respond is hepatic glucose production (second line; HGP declines from 2.0 to 1.34 at the lowest dose):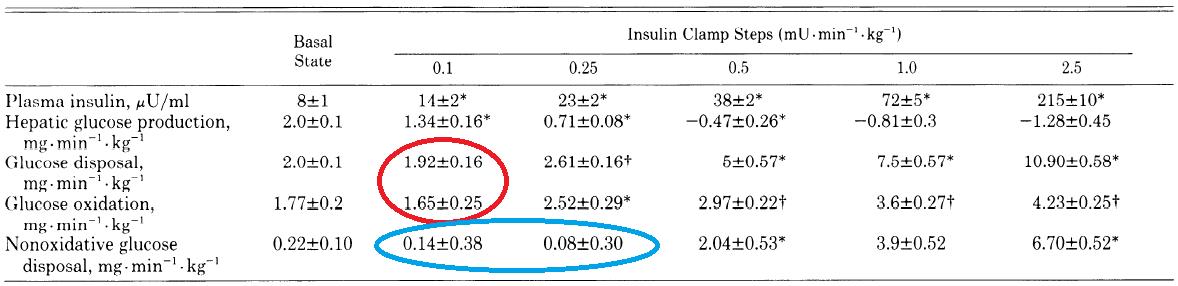
WRT low dose insulin on glucose metabolism: liver responds, not skeletal muscle. Skeletal muscle doesn’t even look at glucose until insulin infusion reaches 250 – 500 uU, which is probably why back in ’64 they saw absolutely no effect at 10 uU. At 100 uU they saw an effect, but according to these data, it was likely due solely to liver, because skeletal muscle doesn’t seem to care until levels exceed 250 uU (it’s an infusion rate, not an absolute concentration. But that’s neither here nor there). To be clear, 10 uU insulin infusion doesn’t affect glucose metabolism (1964). period. 100 uU modestly affects it (1964), and this is probably so modest because only liver is helping out (1990). At 500 uU, full scale attack on blood glucose.
But fatty acids are obliterated with 5 – 50 x less: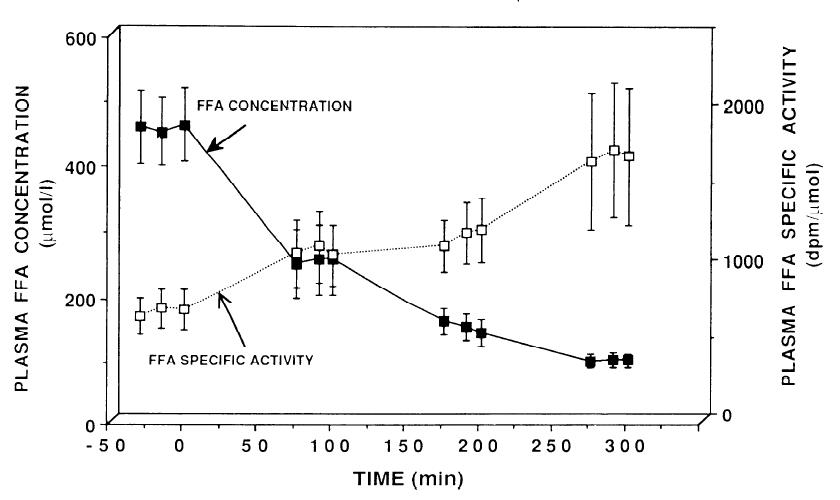
It worked with 10 uU in ’64, and it worked just as well with 100 uU in ’90. (FYI the first paper was published in 1964; this one in 1990).
Furthermore, in the table above glucose metabolism was progressively affected with increasing insulin concentrations. Not so much with FAs:
FA flux is rapidly and completely shut down with a dollop of insulin. Indeed, it is obliterated. Giving more insulin doesn’t do anything, because, well, when you blow down a straw house, it tends to stay down.
calories proper
Become a Patron!
Save
or a vegetarian diet and had their gut microflora analyzed. The low carb diet
was much higher in fat, and as such, increased the prevalence of a microbe involved in fat digestion. “Bilophila.” The article focused on this one and cited a 2012 study where Bilophila was associated with intestinal inflammation… however, the ketogenic diet
increased the levels of Bacteroides and decreased Firmicutes. These are the two that brought the whole gut microbe-obesity connection into the spotlight. The microbiome in obese mice is characterized by low Bacteriodetes and high Firmicutes. Fecal transplants from obese mice to lean mice causes them to gain weight. Little is known about Bilophila relative to Bacteriodetes & Firmicutes, and I suspect the focus was on Bilophila because the authors wanted something negative to say about a meat-based, fiber-free ketogenic diet, and that 2012 mouse study suggested Bilophila could be their answer.