Orlistat blog.
This post is a little long, but the conclusion was bold enough to warrant inclusion of enough studies to independently demonstrate each point. Reducing calorie and dietary fat intake will help weight loss, but it’s not the only way. The goal for this blog entry is to compare the results from studies on Orlistat, which pharmacologically inhibits dietary fat digestion, with the results from low fat and low carb diet studies. In brief, yes, reducing dietary fat and calories pharmacologically via Orlistat or voluntarily via low fat diet causes weight loss, but the metabolic improvements that are usually associated with weight loss are considerably attenuated because of the reduced dietary fat. IOW, for any given amount of weight loss, the metabolic improvements are greater for a diet that restricts carbohydrates (sugars, whole grains, refined grains, cereal fibre, etc.) than for a diet or drug that restricts fats (e.g., low-fat diet, Orlistat, etc.).
Note: Orlistat is biologically similar to a low fat diet. With the low fat diet, dietary fat intake is reduced voluntarily; with Orlistat, dietary fat digestion is reduced pharmacologically. With both, the amount of dietary fat that gets into the body is reduced.
These studies are relatively similar in study design, subject population, duration, and how the interventions were administered. They all lasted at least one year, except the Yancy study, which was only 48 weeks but had to be included because it makes an important connection.
Orlistat (low fat) studies: Krempf, 18 months; Hauptman, 24 months. Low carb vs. low fat: Stern, 12 months; Foster, 12 months. Low carb vs. Orlistat: Yancy: 48 weeks.
Round 1. Orlistat vs. Placebo
Weight reduction and long-term maintenance after 18 months treatment with orlistat for obesity. (Krempf et al., 2003 International Journal of Obesity Related Metabolic Disorders)
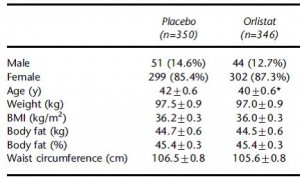
700 obese subjects, baseline characteristics:
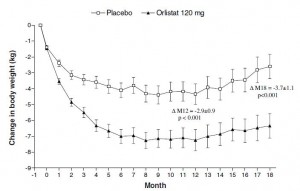
As expected, impressive weight loss in the Orlistat group:
Orlistat group lost 8% of their initial body weight, compared to 3% in the placebo group… Orlistat weight loss was 2.5x greater than placebo.
Important numbers: (Placebo vs. Orlistat)
Fasting glucose: -0.29 mM vs. -0.86 mM
HDL: +31.5% vs. +38.2%
TG: -15.6% vs. -24.4%
Orlistat in the long-term treatment of obesity in primary care settings. (Hauptman et al., 2000 Archives of Family Medicine)
Same basic outline as Krempf study (above). 600+ obese subjects, baseline characteristics:
To compare directly with the Krempf study: by week 76 (18 months), Orlistat group lost 7% of their initial body weight compared to 3% in placebo, just over twice as much. To compare with the rest of the data in this study: by week 104 (24 months), Orlistat group lost 5% of their initial body weight compared to 2% in placebo. Orlistat group lost 2.5x more weight than placebo.
Important numbers: (placebo vs. 120 mg Orlistat [dose used by Krempf])
Fasting glucose: +0.24 vs. +0.16 (yes, fasting glucose actually increased in the Orlistat group)
HDL: +7.7 vs. +5.8% (yes, HDL improved more in the placebo group compared to Orlistat)
TG: -3.0% vs. +13.5% (yes, TGs actually increased in the Orlistat group)
Round II. low carb vs. low fat.
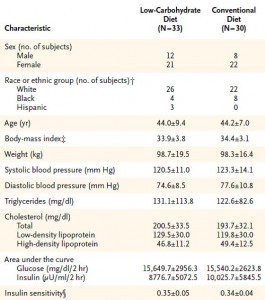
The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. (Stern et al., 2004 Annals of Internal Medicine)
Weight loss:
Low fat dieters lost 2% of their initial body weight, and low carb dieters lost 4%. Although this study was shorter (1 year, compared to 1.5 years in Krempf and 2 years in Hauptman).
Important numbers: (Low fat vs. low carb)
Fasting glucose: -1.11 vs. -1.55
HDL: -12.3% vs. -1.9%
TG: +2.7% vs. -28.2%
http://www.ncbi.nlm.nih.gov/pubmed/12761365
A randomized trial of a low-carbohydrate diet for obesity. (Foster et al., 2003 NEJM)
Baseline characteristics:
Important numbers: (Low carb vs. low fat)
Fasting glucose: ?
HDL: +11% vs. +6.0%
TG: -17% vs. -0.7%
Round III. Low carb diet vs. Orlistat
A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. (Yancy et al., 2010 Archives of Internal Medicine)
This study actually pitted a calorie unrestricted low carb diet directly against Orlistat. Over 100 subjects were included, the details are in line with the above studies.
Body weight: Low carb group, 124 kg -> 113 kg, they lost 9 % of their initial body weight; Orlistat, 119 kg -> 109 kg, they lost 8 % of their initial body weight
Important numbers: (Low carb vs. Orlistat)
Body weight: -9.2% vs. -8.1%
Fasting glc: -9.74 vs. -3.26
HDL: +10.3% vs. +8.7%
TG: -19% vs. -15.7%
Summary
Krempf: placebo vs. Orlistat
Body weight: -3% vs. -8%
Fasting glc: -0.29 vs. -0.86
HDL: +32% vs. +38%
TG: -16% vs. -24%
Hauptman: placebo vs. Orlistat
Body weight: -2% vs. -5%
Fasting glc: +0.24 vs. +0.16
HDL: +7.7 vs. +5.8%
TG: -3.0% vs. +13.5%
Stern: low fat vs. low carb
Body weight: -2% vs. -4%
Fasting glc: -1.11 vs. -1.55
HDL: -12.3% vs. -1.9%
TG: +2.7% vs. -28.2%
Foster: low fat vs. low carb
Body weight: -3% vs. -4%
Fasting glucose: ?
HDL: +6% vs. +11.0%
TG: -0.7% vs. -17%
Yancy: low carb vs. Orlistat
Body weight: -9.2% vs. -8.1%
Fasting glc: -9.74 vs. -3.26
HDL: +10.3% vs. +8.7%
TG: -19% vs. -15.7%
1. In the Krempf Orlistat study, Orlistat caused more weight loss than placebo, and was modestly better at reducing fasting glucose and TGs and increasing HDL.
2. In the Hauptman Orlistat study, Orlistat caused more weight loss but fasting glucose actually increased relative to baseline, the increase in HDL was less than in placebo, and TGs actually increased relative to baseline and placebo.
3. In the Stern low carb study, the low carb diet caused more weight loss than the low fat diet, and the low carb diet lowered fasting glucose modestly better than low fat diet. Changes in HDL and TGs were significantly better in the low carb group.
4. In the Foster low carb study, the low carb group lost modestly more weight than the low fat group, and the changes in HDL and TG were significantly better in the low carb group as well.
5. In the Yancy low carb vs. Orlistat study, the low carb group lost modestly more weight, fasting glucose decreased almost twice as much in the low carb group, and HDL and TG improved significantly more in low carb relative to Orlistat.
Reducing body weight by cutting calories and reducing fat intake (via Orlistat [Krempf, Hauptman, Yancy] or low fat diet [Orlistat studies, Stern, Foster]) consistently produces inferior changes in the metabolic landscape compared to reducing carbohydrate intake (Stern, Foster, & Yancy). Orlistat caused more weight loss compared to placebo (Krempf, Hauptman), but not compared to a low carb diet (Yancy).
Dietary fat increases HDL. Replacing carbs with dietary fat reduces TGs. These things occur independently from weight loss, although weight loss is greater on a low carb diet compared to a low fat diet. IOW, reducing carb intake causes more weight loss and superior changes in risk factor profiles compared to reducing calorie and fat intake regardless of whether fat is reduced via dieting (low fat diet) or pharmacologically (Orlistat).
For full access to all articles and much more (or if you just like what I do and want to support it), become a Patron! Five bucks a month and there are many other options. It’s ad-free and you can cancel if it sucks ????
Also, I’m open to suggestions so feel free to leave a comment or email me directly at drlagakos@gmail.com.
Affiliate discounts: if you’re still looking for a pair of hot blue blockers, Carbonshade is offering 15% off with the coupon code LAGAKOS and Spectra479 is offering 15% off HERE. TrueDark is running a pretty big sale HERE. If you have no idea what I’m talking about, read this then this.
20% off some delish stocks and broths from Kettle and Fire HERE.
If you want the benefits of ‘shrooms but don’t like eating them, Real Mushrooms makes great extracts. 10% off with coupon code LAGAKOS. I recommend Lion’s Mane for the brain and Reishi for everything else.