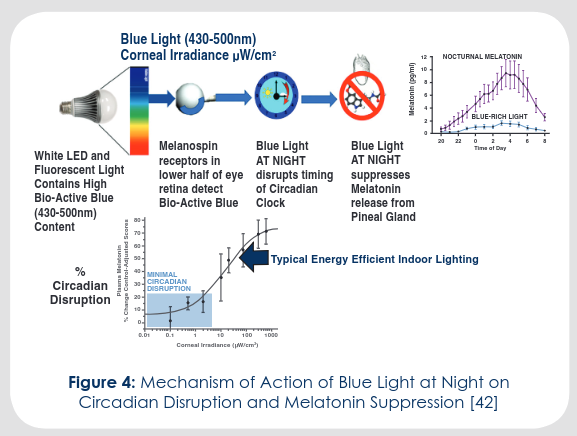
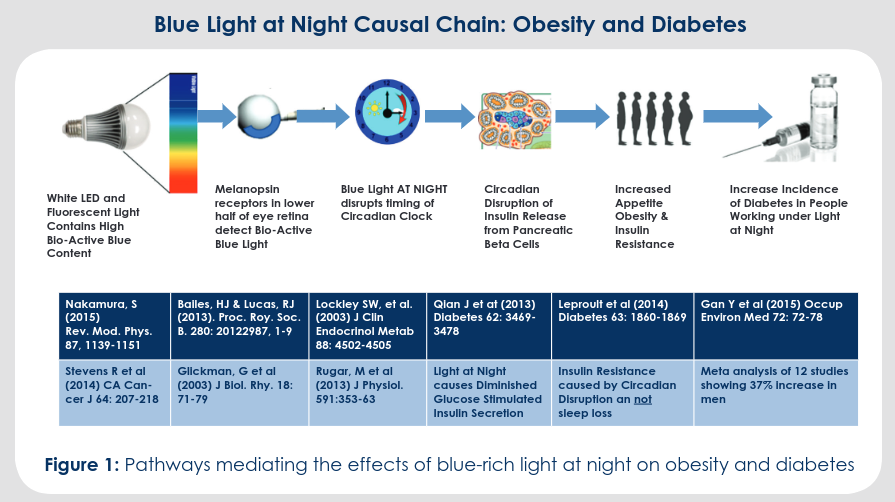
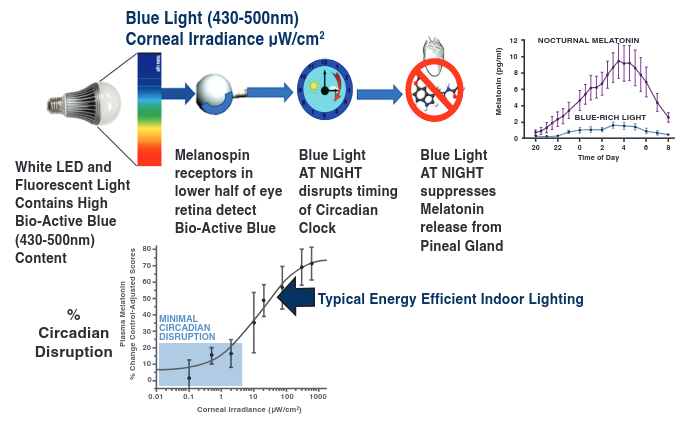
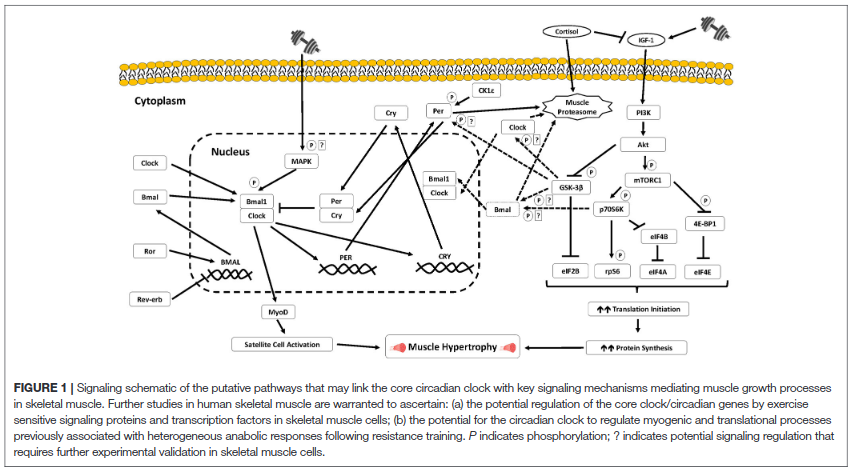
Summary of a presentation by Orie Shafer titled “Circadian timekeeping and entrainment in neuronal clock networks.”
From the classic Wever study, which put participants in underground dungeons to study circadian rhythms in the absence of sunlight…
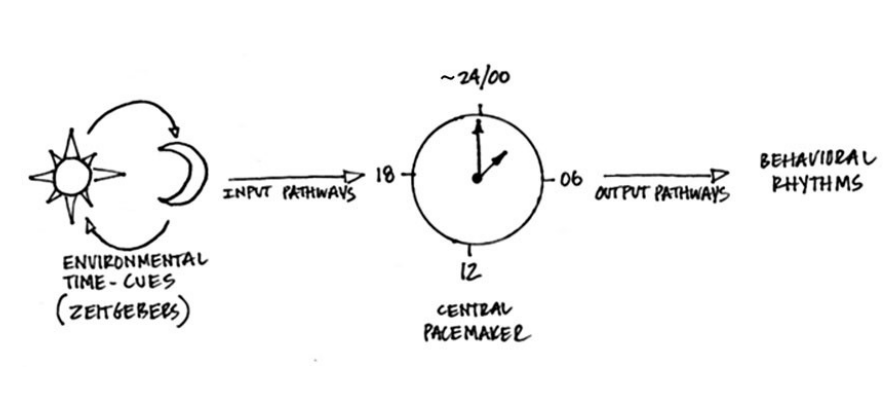
and showed there are multiple inputs, both external and internal zeitgebers.
In this study, in the absence of sunlight, the human circadian rhythm in locomotor activity, bed movements, and rectal temperature was about 25.8 hours:
The study wasn’t perfect, but still cool.
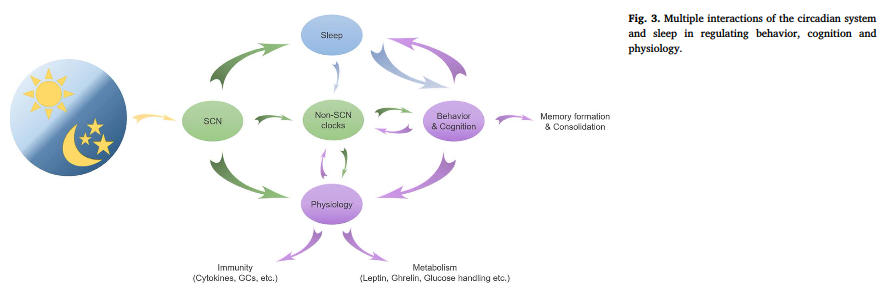
Most people like to simplify and say light is the input, and while it may be the main one, we now know there are also other important inputs such as food intake for the food-entrainable oscillator and exercise for the skeletal muscle clock.
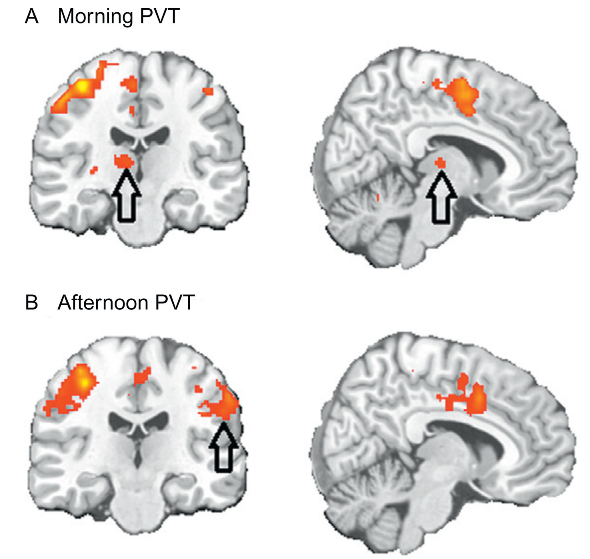
“Behavorial rhythms are driven by molecular rhythms. Molecular clocks are required in small islands of the brain for behavorial, endocrine, and physiological rhythms.” Search for genetically modified models of virtually any clock gene and it’s going to influence a wide variety of processes. Like, circadian rhythms are important for certain aspects of nearly everything. “60% of the time, it works every time” lol
For the rest of this article and I promise it doesn’t suck 🙂 head over to Patreon! Five bucks a month for full access. It’s ad-free and you can cancel at any time.
If you’re interested in setting up consultations with me, reach out: drlagakos@gmail.com.
Affiliate links: Still looking for a pair of hot blue blockers? TrueDark is offering 10% off HERE and Spectra479 is offering 15% off HERE. If you have no idea what I’m talking about, read this then this.
Join Binance and get some cryptoassets or download Honeyminer and get some Bitcoins for free!
20% off some delish stocks and broths from Kettle and Fire HERE.
If you want the benefits of ‘shrooms but don’t like eating them, Real Mushrooms makes great extracts. 10% off with coupon code LAGAKOS. I recommend Lion’s Mane for the brain and Reishi for everything else.
Join Earn.com with this link.
Start your OWN Patreon campaign!