The school lunch program is screwed.
First the USDA modifies the definition of a vegetable to include pizza. Now they significantly altered their standards for school lunches to include fewer healthy foods and more USDA-approved ones (see report at the USDA’s website). In brief, this move further reduces the nutrition of school lunches and will likely do more harm than good. Here’s why:
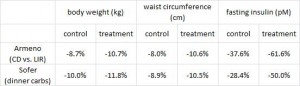
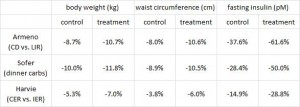
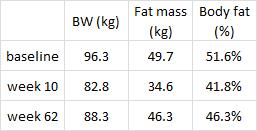
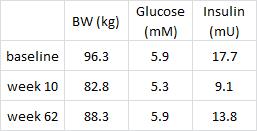
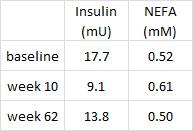
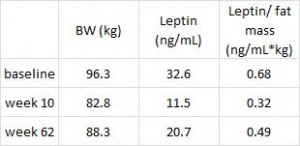
In this cross-sectional Swedish study, parents recorded 7-day food diaries for their 4-year old children who then went in for a regular checkup.
Metabolic markers in relation to nutrition and growth in healthy 4-y-old children in Sweden (Garemo et al., 2006 AJCN)
On a 1,400 kcalorie diet, these children were consuming roughly 15% protein, 33% fat, and 52% carbs (about 20% of which came from sucrose). That seems like a lot of calories, but besides playing all day, 4 year old children are also growing at an incredible rate.
Interesting finding numbers 1 & 2: Children who got most of their calories from fat had the lowest BMI (i.e., they were the leanest), and the opposite was observed for carbs.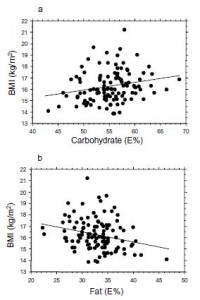
When divided into groups of normal weight vs. overweight and obese, some interesting and non-intuitive patterns emerged. For example, lean kids don’t eat less food; but they do eat fewer carbs and less sucrose (and make up the difference by eating more fat and saturated fat).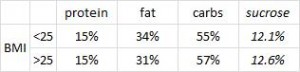
Some of the weaker correlations showed:
-total calorie intake was associated with growth (logical)
-total carbohydrate intake was associated with increased fat mass (unfortunate yet also logical)
-total fat intake was associated with decreased fat mass (interesting)
And those who ate the most saturated fat had the least amount of excess body fat. (more on this below)
Fortunately, in a young child, a poor diet hasn’t had enough time to significantly impact their metabolic health; as such no macronutrient was associated, either positively or negatively, with insulin resistance [yet].
In a more appropriately titled follow-up, Swedish pre-school children eat too much junk food and sucrose (Garemo et al., 2007 Acta Paediatrica), Garemo reported that most of their carbs came from bread, cakes, and cookies, while most of the sucrose came from fruit, juices, jam, soft drinks, and sweets. And WOW, go figure- most of the fat came from meat, chicken, sausage, liver, eggs, and dairy; NOT vegetable oils.
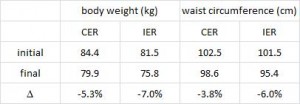
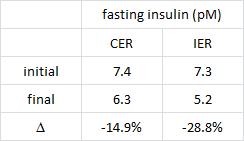
And in a mammoth dissertation, Eriksson (2009) confirmed many of these findings in a larger cohort of 8-year old Swedish children and had this to say about dairy fat: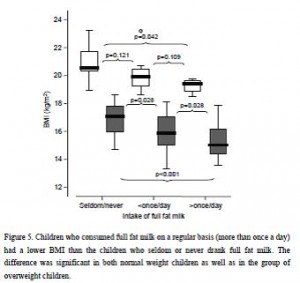
The open boxes represent overweight kids, the closed boxes are lean kids. Going from left to right, in either the open or closed boxes, BMI declines with increasing intake of full fat milk (perhaps parents should reconsider skim milk?). Eriksson also confirmed that saturated fat intake was strongly associated with reduced body weight. Interestingly, she mentioned that food intake patterns are established early in life, so it might be prudent to remove sugars and other nutrient poor carb-rich foods, and introduce nutritious whole foods as early as possible. I’m not exactly sure how she assessed patterns of food intake establishment, but it seems logical. Especially in light of the following study… we’ve seen 4 year olds, 8 year olds, and now we have 12-19 year olds. The relationship between diet and health is consistent across all age groups.
Virtually all of the above data in Swedish children seem to suggest dietary saturated fat, whether it’s from beef, sausage, eggs, whole fat dairy, or liver (i.e., WHOLE food sources; NOT hydrogenated vegetable oils), is associated with reduced fat mass. Metabolic abnormalities were not present, probably because the children were simply too young (although body weight seems to respond relatively quickly, other downstream effects of poor nutrition take years to accumulate before symptoms develop).
An American study about nutrient density and metabolic syndrome was recently published. These kids were exposed to poor nutrition for just long enough to experience some of those malevolent effects.
Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents (Carlson et al., 2011 Journal of the American Dietetic Association)
The figure below divides fiber (a proxy for good nutrition; i.e., leafy vegetables, beans, etc.) and saturated fat into groups of least and most amounts comsumed. The lowest fiber intake was 2.9 grams for every 1,000 kcal, and 9.3% of these kids already had metabolic syndrome; the highest fiber intake was 10.7 grams / 1,000 kcal and 3.2% had metabolic syndrome. Thus, consuming a fiber-rich [nutrient dense] diet is associated with a significantly reduced risk of metabolic syndrome.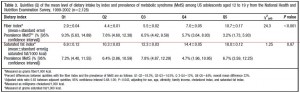
The next rows are saturated fat. The lowest saturated fat intake was 6.9 grams / 1,000 kcal and 7.2% had metabolic syndrome; the highest saturated fat intake was 18 grams / 1,000 kcal and 6.7% had metabolic syndrome…. huh? While it didn’t reach statistical significance, the trend for saturated fat paralleled that of a “nutrient dense” diet. Is it possible that saturated fat might be part of a nutrient dense diet? if saturated fat comes in the form of red meat, liver, eggs, etc., then yes, it is part of a nutrient dense diet. This conclusion evaded both the study authors and the media.
In 4 and 8 year old Swedish children, those who ate the most saturated fat had the least excess fat mass. In 12 – 19 year old American adolescents, those who ate the most saturated fat had the lowest risk for metabolic syndrome.
Is it too much of a stretch to connect these ideas by saying that in the short run, a low saturated fat (nutrient poor, carb-rich) diet predisposes to obesity; and in the long run it predisposes to metabolic syndrome ???
Collectively, these data suggest a diet based on whole foods like meat and eggs, including animal fats, with nutrient dense sources of fiber (e.g., leafy vegetables) but without a lot of nutrient poor carb-rich or high sugar foods, may be the healthiest diet for children. 
Flashback: recap of “USDA vs. nutrition, round I”
USDA: 1
Nutrition: 0
They made pizza a vegetable and insiders suspect that next they’ll try to make it a vitamin.
USDA vs. nutrition, round II
USDA: replacing normal milk with low fat milk
nutrition: full-fat milk was associated with lower BMI in both lean and obese children (see the Eriksson figure above)
USDA: increasing nutrient poor carb-rich options
nutrition: this was associated with increased fat mass in children (Garumen et al., see figures above)
USDA: reducing saturated fat as much as possible
nutrition: reduced saturated fat was associated with excess fat mass in children and metabolic syndrome in adolescents.
Such changes will have an immeasurable long-term impact if children grow up thinking these are healthy options. Finally, this blog post does not contain a comprehensive analysis of saturated fat intake and health outcomes in children, but the USDA’s new regulations should have been accompanied by one. In other words, these regulations should not have been based on the studies discussed above, but the studies discussed above should have been considered when the USDA was crafting their recommendations. Obviously, they weren’t.





 head over to Patreon
head over to Patreon