The eyes are the window through which light must pass, regardless of sightedness.
FACT: we don’t realize the importance of circadian biology. Or at least we don’t act like it. And we’re certainly not going to turn off our iPhones & laptops when we’re supposed to. Potential intervention: hot Blue Blockers. They’re a band-aid, no doubt, but they might help. Jane Plain raised a potential concern with this here. In brief, we can block blue light from molesting circadian biology with hot Blue Blockers, but extraocular light exposure could betray such feeble attempts.
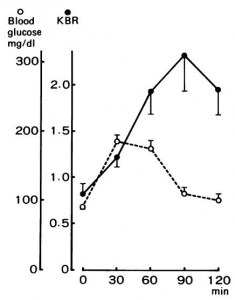
It seems to be based, in part, on an experiment by Campbell & Murphy (1998). They tried to experimentally screw circadia by exposing an isolated spot of skin on the back of the knee to 3 hours of bright light. Melatonin data weren’t shown, but the authors said they mirrored body temperature:
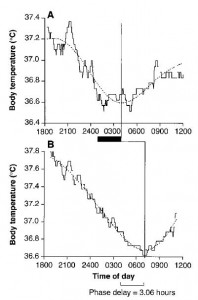
It worked (for body temperature, at least).
But FAR more interestingly, Czeisler showed bright light-induced melatonin suppression in blind people was reversed if they covered their eyes!!!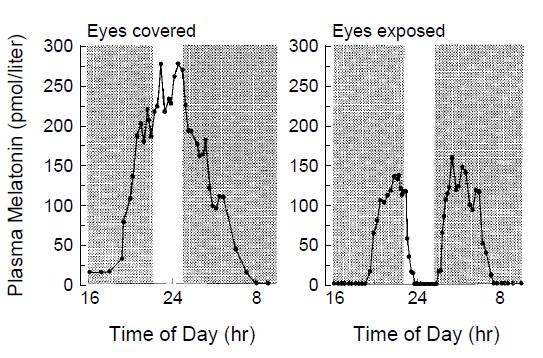
This is wild. Unless there is something CircadianlyMagical about the skin on the back of the knees, then these findings refute those of Campbell. Czeisler’s findings were confirmed by Hatonen (1999) in sighted people: black circles = no light exposure; open squares = full-face light exposure with eyes closed (partially blunted melatonin secretion); and open circles = full-face light exposure with eyes open (fully blunted melatonin):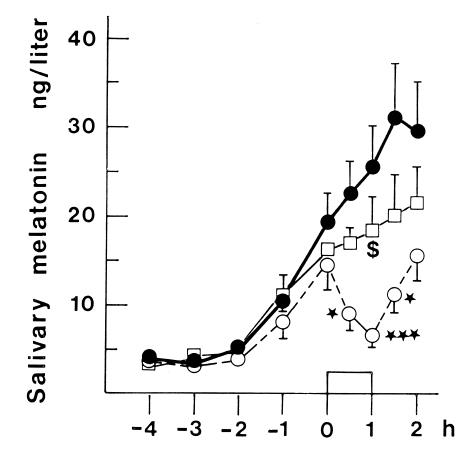
Of note, blind eyes and closed eyes aren’t the same as covered eyes. There were, however, 2 people who exhibited no melatonin inhibition with closed eyes. Perhaps some are intrinsically more light-resistant, or have robust eyelids or something.
It seems as though we needn’t worry about Campbell’s findings after all because they were directly refuted by Hebert (1999):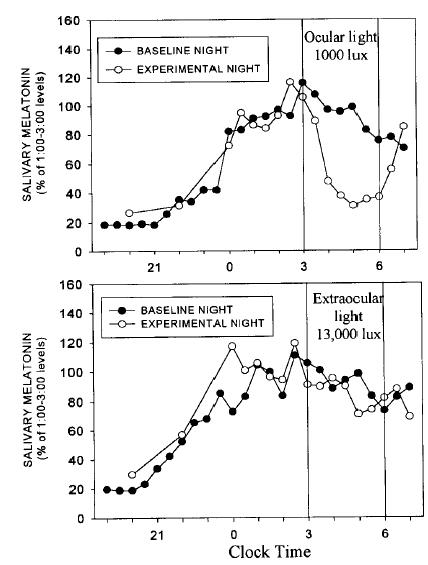
The light exposure protocol in both of the studies was identical: 13000 lux to the back of the knees for 3 hours.
Perhaps we should’ve demanded to see Campbell’s melatonin data? Or not. Lushington confirmed Hebert’s findings (albeit with only 11000 lux):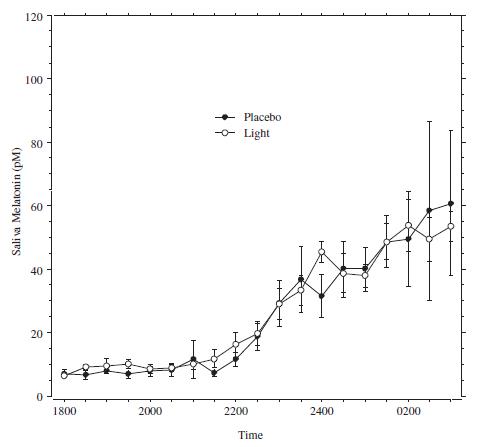
In 2000, Lindblom blasted 10000 lux at a much larger surface area – chest & abdomen – and found no effect on melatonin: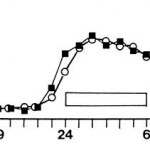
The eyes are the window through which light must pass, regardless of sightedness.
Was all of this blog post irrelevant until now? Maybe. (sorry)
Sasseville compared bright light-induced melatonin suppression in people wearing boring shades (top graph) or hot Blue Blockers (SolarShield Orange Lenses ) (bottom graph):
) (bottom graph):
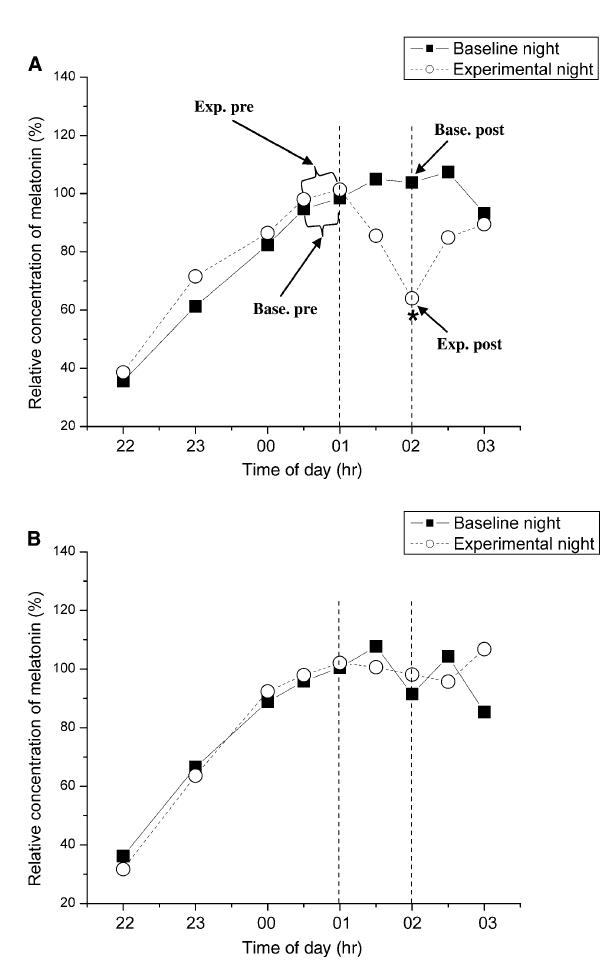
The orange lenses transmit slightly less light than the boring ones (32 vs. 52%), so they accounted for this by hitting the hot Blue Blockers with more lux (4000 vs. 2200… this is directly in their faces, so it couldn’t be >10000 lux like in the previous studies)… this still resulted in more irradiance hitting the hot Blue Blockers, so the odds were stacked against them (I think, #physics).
Lux: luminous flux per unit area
Irradiance: electromagnetic radiation per unit area
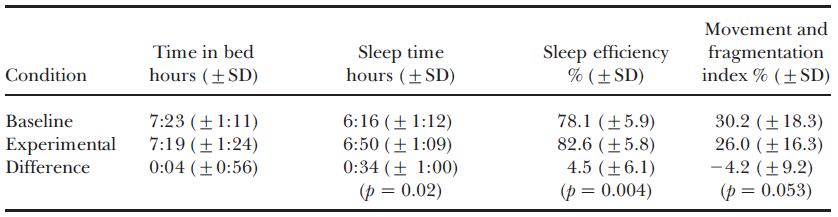
Melatonin suppression is important, but what we’re really talking about here is SLEEP. And in 2009, Burkhart showed just that. When assigned to hot Blue Blockers (NoIR Polycarbonate Lasershields), sleep quality markedly improved:
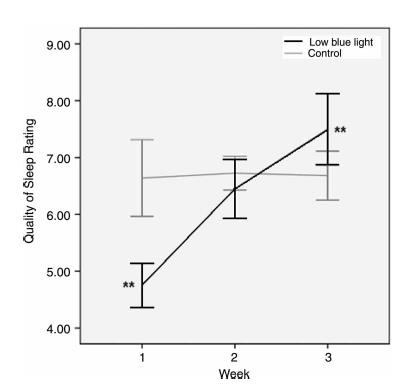
(granted, randomization was horribly bollixed, but it is what it is).
Sasseville came through again in 2009, this time for shift workers. Their subjects had to wear hot Blue Blockers (Uvex Skypers) when they were leaving work [in the morning]. It worked.
when they were leaving work [in the morning]. It worked.
In sum, don’t sweat extraocular light exposure, and anyone with a metabolic disturbance who lives a remotely modernized existance, paleo or otherwise, might benefit from these.
Flash forward to 2017… Circadian rhythms: blocking the blues. AND THE GREENS
calories proper
Become a Patron!
hot Blue Blocker experiment: expectations = none. I’m a “non-responder.” This might not be the best time of year to conduct such an experiment, but the combination of high motivation and low patience prevailed. And I still use my computer a lot at night.
This is a diary of sorts.
day 1: initial observations.
Started wearing them about 2 hours before sunset. Outside sky prior to dusk looks like insane alien invasion. But creepy red bathroom light looks exactly the same. #physics.
Morning of day 2: Usually wake once or twice in the middle of the night, but didn’t…
Evening of day 2: Started rocking the shades 2 hrs prior to bedtime. Same awesome yellow-ness and crisp resolution of the sunset. It really looks like another planet. I also could’ve probably stared directly at the sun with impunity (but didn’t).
Morning of day 3: new conclusion: I think I usually wake up a few hours prior to dawn, but hot Blue Blockers prior has shifted this to a few hours earlier.
Morning of day 4: same! Hot Blue Blockers make me need to pee 4 hours sooner after falling asleep <– I’m a “responder!”
Mood, sleep quality, & energy levels stable <– “non-responder,” but willing to give it more time. Burkhart’s study showed a near doubling of sleep quality, but it took 3 weeks.
P.S. FWIW, I’m wearing these , so definitely not going out in public places.
, so definitely not going out in public places.
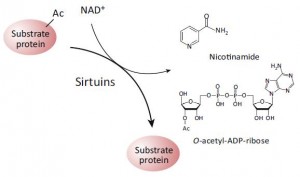
(which may or may not be acting through SIRT1; this is controversial). While alcohol does no great favors for circadian biology, if you’re going to imbibe, perhaps a resveratrol-rich Argentinian malbec served, and this might be the important part, at night, when all of this stuff is going on… coincidentally [fortunately], that’s precisely when most choose to imbibe.