Part I: Circadian Mismatch
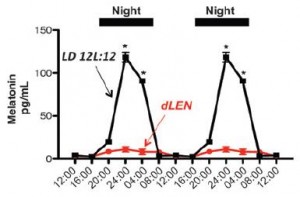
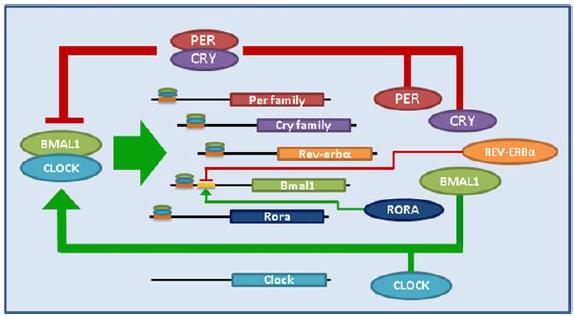
1. Artificial light at night suppresses melatonin (Lewy et al., 1980); induces “circadian mismatch.”
2. Circadian mismatch is associated with and/or predisposes to breast cancer (eg, He et al., 2014 and Yang et al., 2014).
3. In this epic study, human breast cancer xenografts were exposed to blood taken from healthy, pre-menopausal women during the day (melatonin-depleted), at night (high melatonin), or at night after light exposure (melatonin-depleted) (Blask et al., 2005). They showed that tumors exposed to melatonin-depleted blood exhibited higher proliferative activity than those exposed to melatonin-repleted blood. This has been deemed one of the most “ethical” studies to demonstrate a causal link between circadian mismatch and cancer.
4. And to make matters worse, circadian mismatch also reduces the efficacy of cancer drug therapy (Dauchy et al., 2014). This study showed that, in a rodent model of breast cancer, exposure to light at night (circadian mismatch) enhanced tumor development and induced tamoxifen-resistance, and this was abolished by melatonin replacement.
They also suggested a mechanism: tumors metabolize linoleate into the mitogen 13-HODE. Melatonin suppresses linoleate uptake.