Part I: Circadian Mismatch
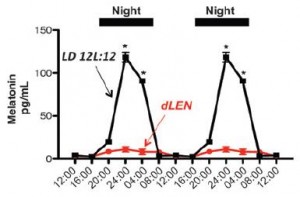
1. Artificial light at night suppresses melatonin (Lewy et al., 1980); induces “circadian mismatch.”
2. Circadian mismatch is associated with and/or predisposes to breast cancer (eg, He et al., 2014 and Yang et al., 2014).
3. In this epic study, human breast cancer xenografts were exposed to blood taken from healthy, pre-menopausal women during the day (melatonin-depleted), at night (high melatonin), or at night after light exposure (melatonin-depleted) (Blask et al., 2005). They showed that tumors exposed to melatonin-depleted blood exhibited higher proliferative activity than those exposed to melatonin-repleted blood. This has been deemed one of the most “ethical” studies to demonstrate a causal link between circadian mismatch and cancer.
4. And to make matters worse, circadian mismatch also reduces the efficacy of cancer drug therapy (Dauchy et al., 2014). This study showed that, in a rodent model of breast cancer, exposure to light at night (circadian mismatch) enhanced tumor development and induced tamoxifen-resistance, and this was abolished by melatonin replacement.
They also suggested a mechanism: tumors metabolize linoleate into the mitogen 13-HODE. Melatonin suppresses linoleate uptake.
Part II: Chronopharmacology
…more questions than answers
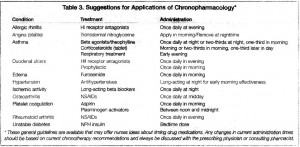
Taking uppers in the morning and downers at night is obvious. Why isn’t this established for more drugs?
Disclaimer: fixing circadian mismatch should be prioritized, numero uno. But some people still need drugs; if superior circadian dose timing allows for dose reductions and thus fewer side effects, I’m all for it.
Metformin used to be considered a dirty drug (ie, unknown or multiple targets), but the most likely target is AMPk, which inhibits the gluconeogenic enzyme PEPCK. Doesn’t cause much hypoglycemia, but prevents hyperglycemia. An interesting [preliminary] rodent study showed that metformin also induces circadian phase advance (Um et al., 2007)… 1) Does it matter what time of day metformin is administered? Seasonally? 2) Circadian mismatch is often caused by excess light at night = phase delay. Is part of metformin’s efficacy due to countering this?
Another study showed that metformin increases the amplitude in circadian gene expression (Barnea et al., 2014). Amplitude is blunted during circadian mismatch. Is part of metformin’s efficacy due to countering this?
Thiazolidinediones are anti-hyperglycemic and anti-inflammatory; they cause weight gain but are otherwise effective. Interestingly, one study showed that rosiglitazone directly targets Bmal1, a circadian transcription factor (Wang et al., 2008). 1) Does it matter what time of day rosiglitazone is administered? 2) Is part of rosi’s mechanism via Bmal1 activation?
Some pro-inflammatory cytokines exhibit a circadian peak in the early morning hours. This is thought to worsen symptoms in some patients with rheumatoid arthritis in the morning, and one study showed that taking prednisolone (a synthetic glucocorticoid that suppresses inflammation) at 2 am, prior to the cytokine peak, was more effective than taking it at 7:30 am (Arvidson et al., 1997)… inconvenient, but could allow for dose-lowering.
Asthma follows the opposite rhythm: worse at night (in some patients). Fortunately, some anti-asthmatic medications are already recommended to be dosed at night.
Blood pressure is supposed to dip at night, although this doesn’t happen in some hypertensive patients and this is thought to be of pathological importance. Other hypertensives experience an exaggerated peak in blood pressure in the early morning. Should their anti-hypertensive medication be dosed at night? Researchers have already figured out that sustained-release meds taken at night solve these problems for some patients (eg, White et al., 1995 and 1997).
Bromocriptine, a dopamine agonist, reduces 24-hour blood glucose levels if taken immediately upon wakening… but people probably do this already (AM is the most common dose time for all drugs).
Urinary pH regulates renal excretion of some drugs; it’s lowest in the morning – this should be taken into account.
Some people are larks, others are owls. Does this effect PK/PD or drug efficacy? My guess is yes, at least for some drugs, because, for example, it’s been shown that ACTH peaks almost 4 hours earlier in ‘morning people’ compared to ‘night people’ (6 am vs. 10am).
Chronopharmacology: new insights and therapeutic implications
Circadian rhythms and medical diseases: does it matter when drugs are taken?
^^^This paper has an awesome table showing dose-timing vs. efficacy on a great number of drugs. They concluded: “Adequate evidence seems to support that at least ACE-inhibitors or ARBs, statins, corticosteroids (slow-release formulation) for arthritic patients, and ranitidine should preferably be administered in the evening. Morning dosing could be better for proton pump inhibitors, whereas time of administration is not crucial for asthma inhalation drugs.”
(needless to say, findings are mixed at this stage in the game)
Medication timing for the elderly: the impact of biorhythms on effectiveness
Statins work better when taken at night – possibly because this is when endogenous cholesterol synthesis peaks. I’m not pro-statin, but if better dose-timing leads to lower dose requirements and cuts down on nasty side effects, then I’m all for it. But really, I just mention TZDs and statins as examples of how circadian rhythms could impact drug dosing and/or metabolism, not reasons to take these drugs.
What about in people on low carb diets or intermittent fasting regimens, or night shift workers? All. Bets. Are. Off. This likely highly changes how drugs are metabolized, and they’d need to be re-tested in this context. Oh well.
Check out my Patreon campaign! Five bucks a month for full access and there are many other options. It’s ad-free and you can cancel if it sucks ????
Consults are open, contact me if you’re interested: drlagakos@gmail.com
Affiliate links: It’s 2018, join Binance and get some cryptoassets ordownload Honeyminer and get some Bitcoins for free!
Still looking for a pair of hot blue blockers? Carbonshade and TrueDark are offering 15% off with the coupon code LAGAKOS and Spectra479 is offering 15% off HERE. If you have no idea what I’m talking about, read this then this.
20% off some delish stocks and broths from Kettle and Fire HERE.
If you want the benefits of ‘shrooms but don’t like eating them, Real Mushrooms makes great extracts. 10% off with coupon code LAGAKOS. I recommend Lion’s Mane for the brain and Reishi for everything else.
Join Earn.com with this link.