The faux-low carb mouse
Hyperinsulinemia drives diet-induced obesity blah blah blah (Mehran et al., 2012)
The researchers generated a mouse with half as much insulin as normal mice. Physiological insulin levels remain intact, but hyperinsulinemia is genetically inhibited. For the sake of simplicity, we’ll call them “InsKO.”
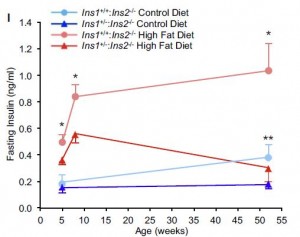
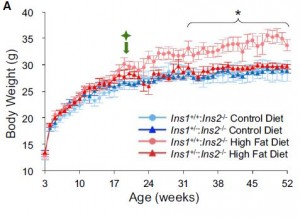
When fed a high fat diet, normal mice become markedly hyperinsulinemic (pink line) whereas InsKO mice maintain relatively normal insulin levels (red line). Blue lines are chow-fed mice; similar trend but less interesting.
because all along they’re eating just as much but burning a whole lot more: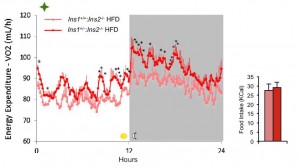
“they’re eating just as much but don’t get fat because there’s not enough insulin”
Adipose tissue is resistant to insulin in the normal mice, as demonstrated by increased free fatty acids (pink bar below) despite higher insulin levels (pink line above). The opposite is true for InsKO mice: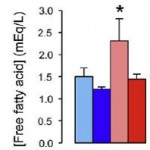
But this doesn’t matter so much because InsKO’s chronically lower insulin levels never allow excess fat to be stored in the first place. As they overeat a high fat diet, the fat can’t get stored so it’s burned instead.
Diatribe, part I.
I call them the faux-low carb mice because similar to humans on a low carb diet: 1) insulin levels are low; and 2) energy expenditure is high. In the infamous Ebbeling study (discussed here: Missing: 300 kilocalories), energy expenditure in weight-reduced patients assigned to the low carb diet
was ~300 kcal higher than those assigned to the isocaloric low fat diet
(“isocaloric,” just like the faux-low carb mice); this is true in a variety of contexts (eg, Metabolic rate per se).
But the InsKO model is much more nuanced…
For example, you need insulin to store fat; InsKO mice don’t have it. But insulin sensitive adipocytes store more fat; InsKO mice have it. But insulin sensitive adipocytes need more insulin to store fat; InsKO mice don’t have it.
Fat tissue in obesity keeps growing (because of the carb-induced hyperinsulinemia) until adipose becomes insulin resistant, at which point or shortly thereafter on comes frank type II diabetes, then later some of the rather nasty complications.
The Hao super diet study (discussed here: Insulin per se) showed elevated insulin levels, whether caused by feeding glucose, sucrose, high GI starch, or glybenclamide, increase fat mass, whereas lowering insulin with nifedipine reduces fat mass. Diazoxide, octreotide, and low carb diets reduce insulin and fat mass whereas insulin therapy increases both (discussed here: Insulin, the nutrient anti-partitioner; and here: an elusive rogue criminal mastermind).
The constant access to dietary and stored fat in InsKO mice, via low insulin levels, causes elevated energy expenditure which provides an “out” for the fat to be burned away. This is similar to the PPARg+/- mice who have an inability to store fat because of a defect in adipogenesis, leading to increased energy expenditure (Kubota et al., 1999):
Whether it is via reduced insulin levels (InsKO & low carb diets), reduced insulin secretion (diazoxide, octreotide, nifedipine), or reduced adipogenesis (PPARg+/-), an inability to store fat causes higher fat oxidation and lower body weight. Remember: “[InsKO mice are eating just as much but don’t get fat because there’s not enough insulin.” On the flipside, increased ability store fat via insulin injections (type I diabetes) or increased adipogenesis (PPARg agonists like the antidiabetic drug rosiglitazone) cause increased adipose growth.
Diatribe, part II.
InsKO mice exhibit increased energy expenditure, which could account for their leanness, discounting a role for reduced insulin levels per se (or not). So which is it: energy expenditure or insulin levels?
Existence of the fat-but-fit obese exercisers (eg, Ortega et al., 2012 and Pajunen et al., 2011) tell us that increasing energy expenditure alone can counter metabolic dysregulation while leaving obesity unscathed (they’re “FAT” but fit). Yes, insulin levels decline, but obesity remains because the primary intervention, exercise, improves metabolism but forces hyperphagia which inhibits the attainment of leanness. InsKO mice have increased energy expenditure AND “relative” hyperphagia (“relative” to body weight; “they’re eating just as much [on an absolute basis] but don’t get fat because there’s not enough insulin“), but not obesity. When the primary intervention involves increased energy expenditure, obesity remains. When the primary intervention involves decreased insulin levels, obesity goes away.