The Randle Cycle, put forth in 1963, dictates that increased fatty acid oxidation inhibits glucose uptake and increased glucose oxidation inhibits fatty acid oxidation – it just makes sense. Insulin enhances glucose uptake and oxidation while suppressing lipolysis; growth hormone, cortisol, and adrenaline enhance lipolysis and fatty acid oxidation which suppresses glucose oxidation. Low carbohydrate diets reduce insulin, and the reduced glucose oxidation is metabolically irrelevant because of reduced glucose intake (by definition). This is critical information. And as a student of basic intermediary metabolism, I prefer the Randle Cycle over any number of alphabet soup recipes to explain metabolic phenotypes (eg, fat and carbs as opposed to IRS, Akt, Jnk, ERK, etc., etc.). Many valuable lessons can be learned from understanding permutations of the Randle Cycle.
For example,
Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice (Keung et al., 2012)
divide and conquer
Part 1. Insulin sensitivity
While it’s weird to think that blocking fatty acid oxidation would have any positive effects, given the theory put forward by Sir Randle, we could guess that it would ramp up whole body glucose oxidation tremendously. And it follows that glucose tolerance and insulin sensitivity would improve. Oxfenacine inhibits fatty acid oxidation and indeed, this is the case: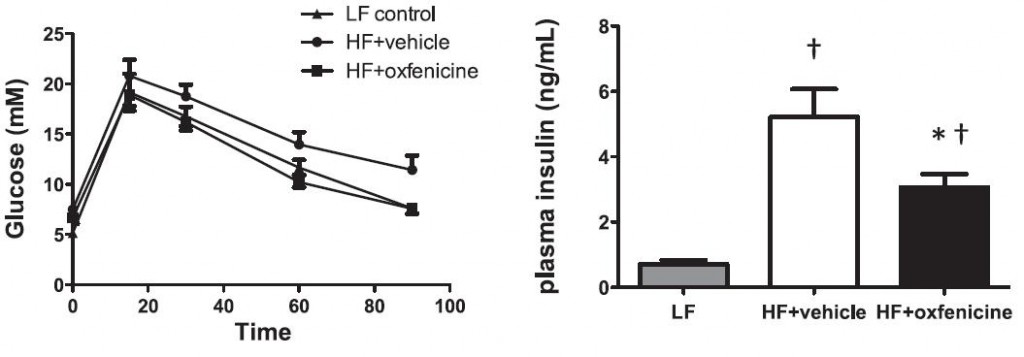
Boooring.
Part 2. The Randle Cycle vs. Laws of Energy Balance
Decreased fatty acid oxidation = increased glucose oxidation and weight loss?
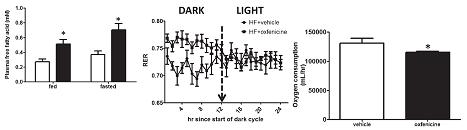
 Oxfenicine reduces insulin, leading to enhanced lipolysis, but all those plasma FFAs had nowhere to go since they couldn’t be oxidized. This is evidenced by: 1) elevated FFAs (figure below on the left); and 2) increased RER (figure below in the middle).
Oxfenicine reduces insulin, leading to enhanced lipolysis, but all those plasma FFAs had nowhere to go since they couldn’t be oxidized. This is evidenced by: 1) elevated FFAs (figure below on the left); and 2) increased RER (figure below in the middle).
side note 1. elevated plasma FFAs don’t always cause insulin resistance.
side note 2. oxfenicine is relatively selective toward skeletal muscle. So liver fatty acid oxidation continues; maybe this is why they weren’t eating more to compensate for the reduced energy availability? Ie, there wasn’t reduced energy availability; muscles oxidized more glucose and the liver oxidized more fat (as evidenced by a slightly smaller liver [1.49 vs. 1.28 grams]). Capiche?
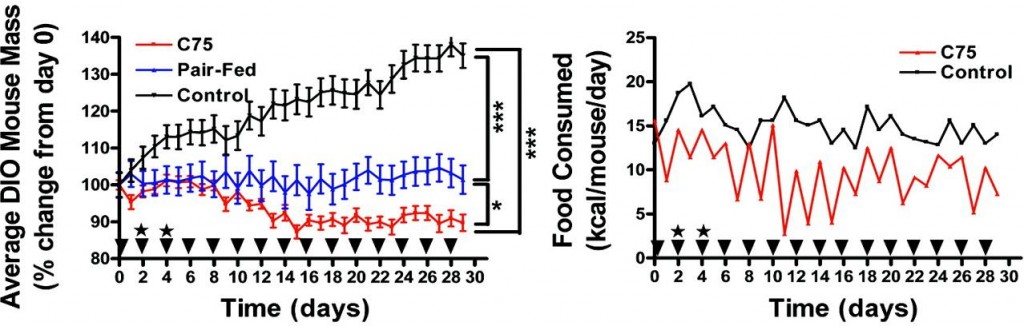
Etomoxir, a nonselective fatty acid oxidation inhibitor, does not have this effect and therefore decreases energy expenditure (left, Thupari et al., 2002) while increasing food intake (right, Horn et al., 2004).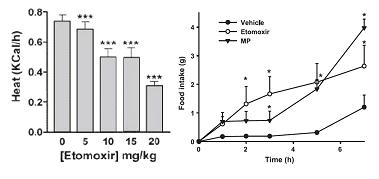 And C75, an indirect enhancer of fatty acid oxidation, reduces body weight and food intake, AND increases energy expenditure (Thupari et al., 2004).
And C75, an indirect enhancer of fatty acid oxidation, reduces body weight and food intake, AND increases energy expenditure (Thupari et al., 2004).
Collectively, increased fatty acid oxidation is good (duh [eg, C75]), reduced fatty acid oxidation is bad (duh [eg, etomoxir]), and reduced fatty acid oxidation selectively in muscle is ambiguously neutral (huh? [eg, oxfenicine]). Increased fatty acid oxidation, at least in the liver, tells the brain there is sufficient energy available, so its OK to stop grazing. Insulin prevents this from happening (thank you, Sir Randle).
Oxfenicine-treated mice lost weight, but oxygen consumption, a surrogate for energy expenditure, went down too. And if we are to take their word for it, food intake, physical activity, and heat production were unchanged.
side note 3a. This finding exposes a flaw in the use of oxygen consumption as a surrogate for energy expenditure. Biochemically speaking, fatty acids are less oxidized than glucose. So to get the same amount of energy from fatty acids requires more oxygen. On the flipside, to get the same amount of energy from glucose requires less oxygen. THIS is what oxfenicine does: similar heat production from less oxygen because oxfenicine forces muscles to oxidize glucose.
Side note 3b. This is tangentially related to why some lung disease patients are advised to follow a low glucose (high fat) diet. Burning glucose produces more CO2 to get the same amount of energy compared to burning fat. Reducing the amount of CO2 you need to exhale is easier on the lungs.
Wow, this post is just full of nutrition science tidbits 🙂
BUT, no change in food intake or energy expenditure, and decreased body weight??? My Spidey sense is going wild. Could this challenge the Laws of Energy Expenditure?
Where’d the fat go? There were increased free fatty acids in the blood, but they still have weight and might affect adipose mass but not body weight. Furthermore, even though plasma free fatty acids were doubled, this would likely amount to something on the order of < 100 ug, compared to the 10,000,000 ug of fat in adipose (in other words, increasing the release of fatty acids from adipose would have no appreciable effect on adipose tissue mass). POST YOUR GUESSES! I’m almost always the first one to attribute fat loss to reduced insulin levels, but it usually has a reason other than magic or gnomes. And I’m sure it does in this case too.
I’m hoping this isn’t the answer: the reduction in body weight was reduced by a statistically barely-nonsignificant amount (p=0.13). In other words, it went down but the study wasn’t powerful enough to statistically detect the difference. I’m hoping the same thing didn’t happen to food intake and heat production, because, well, “data not shown.”
P.S. ambulatory activity was shown you clowns. Figure 2B, remember?