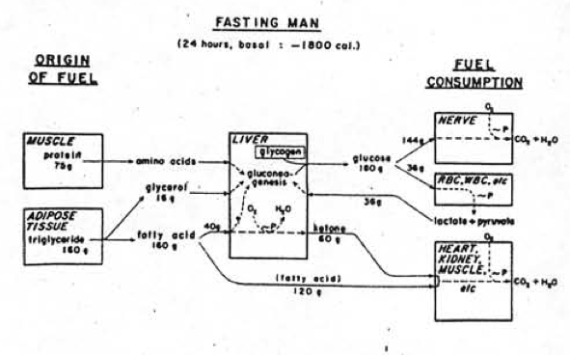
We’ve had a couple more studies on the various ketone supps: two esters (D-b-hydroxybutyrate-R 1,2-Butanediol Monoester and R,S-Butanediol Diacetoacetate) and one beta-Hydroxybutyrate sodium and potassium salts (KetoForce). We’ll call them Clarke, Burke, and Stewart so I don’t get Carpal Tunnel Syndrome typing them out each time. Technically, b-hydroxybutyrate isn’t really a ketone, but whatevs.
[Patron link]

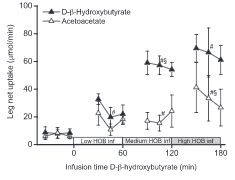
“Clarke”

“Burke”

“Stewart” (this is actually a blend of the sodium “Na” and potassium “K” [not shown] salts)
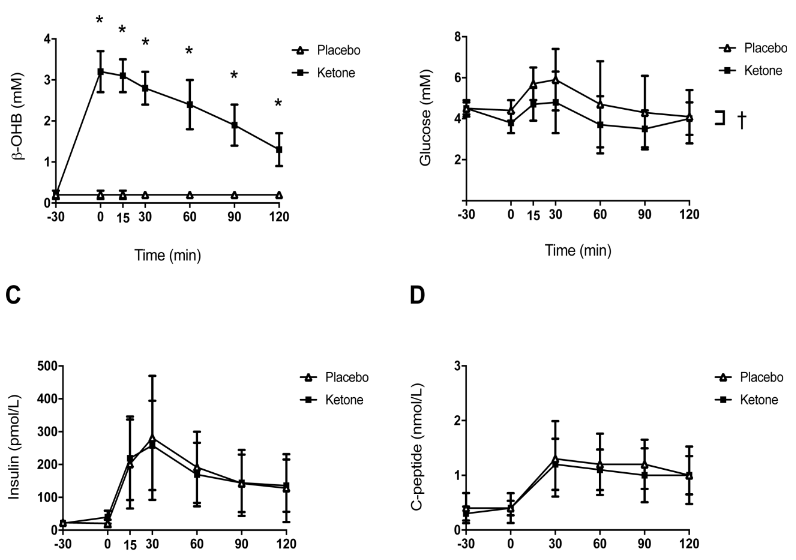
Two studies on Stewart
Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males (O’Malley et al., 2017)
“Ten healthy, recreationally active men.” The participants did a brief warm-up then a 150 km cycling time trial after receiving 24 g Stewart or salt-matched placebo. Controlling for salt was cool, but Stewart has about as 5 kcal/g, so the placebo could’ve controlled for that somehow, with either fat or carb, or something… (probably wouldn’t have mattered anyway)
Results: blood beta-hydroxybutyrate reached about 1 mM, power output declined 7% and it took the keto group about 45 seconds longer to complete the time trial.
Oral beta-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise (Rodger et al., 2017)
“Highly trained cyclists.” Similar study design as McSwiney’s — drain the tank with 90 minutes cycling at 80% max then 4 minute maximal performance test. Same dose as above in 2 divided doses. Same issue with the control group.
Results: blood beta-hydroxybutyrate reached ~0.6 mM and there was a trivial (non-significant) increase in power. This is actually in line with my interpretation of McSwiney regarding the decline in power output before and after draining the tank; ketoadaptation and all that jazz.
One study on Burke
Ketone diester ingestion impairs time-trial performance in professional cyclists (Leckey et al., 2017)
“Internationally competitive elite cyclists.” Two doses of 20 g Burke then a 20-minute warm-up followed by a 31 km time-trial. Non-caloric placebo control, whereas Burke is estimated 4.7 kcal/g. They were well-fed and caffeinated.
Results: beta-hydroxybutyrate reached ~1.1 mM, time-trial took 2% longer, and power was reduced 3.7%.
One study on Clarke
Nutritional ketosis alters fuel preference and thereby endurance performance in athletes (Cox et al., 2016)
“High performance athletes.” This study was different: one group got a drink that was 40% Clarke and 60% carbs (by calories), the other drink was 100% carbs. Calorie-controlled. They cycled for an hour at 75% max (to drain the tank), then 30-minute time trial.
Results: this is the first one that worked. beta-hydroxybutyrate reached 2-3 mM and they made it 2% further during the time-trial.
Affiliate discounts: if you’re still looking for a pair of hot blue blockers, Carbonshade is offering 15% off with the coupon code LAGAKOS and Spectra479 is offering 15% off HERE. If you have no idea what I’m talking about, read this then this.
20% off some delish stocks and broths from Kettle and Fire HERE.
If you want the benefits of ‘shrooms but don’t like eating them, Real Mushrooms makes great extracts. 10% off with coupon code LAGAKOS.
For my interpretation of these studies and an explanation why I think the different keto supps had different effects (or if you just like what I do and want to support it), head over to Patreon! Five bucks a month for full access and there are many other options. It’s ad-free and you can cancel if it sucks 🙂
Also, I’m open to suggestions so feel free to leave a comment or email me directly at drlagakos@gmail.com.
Affiliate discounts: if you’re still looking for a pair of hot blue blockers, Carbonshade is offering 15% off with the coupon code LAGAKOS and Spectra479 is offering 15% off HERE. TrueDark is running a pretty big sale HERE. If you have no idea what I’m talking about, read this then this.
20% off some delish stocks and broths from Kettle and Fire HERE.
If you want the benefits of ‘shrooms but don’t like eating them, Real Mushrooms makes great extracts. 10% off with coupon code LAGAKOS. I recommend Lion’s Mane for the brain and Reishi for everything else.
calories proper
Become a Patron!