Fish blog, take I
Eat fish people. No, don’t eat “fishpeople,” … nevermind. I am a strong proponent of eating salmon so this blog was created to figure out which is the best kind to eat. Priorities are 1) least toxins and 2) best fatty acid composition.
Round 1. Metals in salmon: Farmed vs. wild
A survey of metals in tissues of farmed Atlantic and wild Pacific salmon (Foran et al., 2004)
Farmed Atlantic salmon were sourced from North American commercial suppliers and included salmon from British Columbia, Chile, Maine, and Norway. They got 10 fish from 3 different suppliers in each region: 4 regions x 3 suppliers/region x 10 fish = 120 fish. Species: Farmed Atlantic (didn’t realize that was a species… this is one of those “a-duh” moments).
Wild salmon were from suppliers in Alaska, British Columbia, and Washington for a total of 6 batches of 10 fish. Species: chum & coho.
Methods: BORing
Results:
Divide and conquer.
The amount of metals in Farmed Atlantic (filled bars) and wild salmon (open bars):

Co, cobalt; Cu, copper; Sr, strontium; Cd, cadmium; Pb, lead; U, uranium; As, arsenic; MeHg, methylmercury.
The authors noted some statistically significant differences in cobalt, copper, cadmium (all modestly higher in wild salmon), and the nontoxic “organic” arsenic (higher in farmed salmon), but while those differences may be significant statistically, they don’t look significant physiologically. (if the authors wanted to make the differences look bigger, perhaps they should have opted for a linear ordinate; or maybe they just wanted to squeeze everything in one figure instead). Interestingly, similar levels of these metals were found regardless of where the salmon came from. I would’ve imagined a Farmed Atlantic salmon from Norway would be vastly different than a Farmed Atlantic from British Columbia. Guess not.
As expected, mercury content was correlated with body size (higher up on the food chain, more mercury accumulation), but this is pretty much meaningless to the consumer because we have no idea of the fish’s weight when it was intact. IOW, the salmon on the bottom (figure below) would have less mercury than the one on the top,

But I have no way of knowing who these came from (salmon fillets):

Fortunately, salmon is a relatively “clean” fish, so it doesn’t really matter.
Back to the data.
Oddly, the authors noted that wild salmon were longer than Farmed Atlantic, but mercury content didn’t correlate with length, only body size (fatness? muscularity? weird).
acceptable levels for metals in fish:
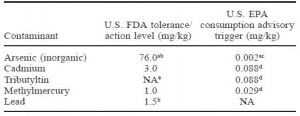
Farmed Atlantic and wild (Coho & Chum) salmon were equivalent and well beneath both the FDA and the far more stringent EPA’s limits. On a side note, I learned that the FDA allows a higher amount of contaminants because they are talking about exposure to each contaminant individually. The EPA is stricter because they are taking into consideration the fact that we are exposed to multiple contaminants simultaneously (“toxic world,” and all that jazz). For example, you would be safe consuming a fish with 76.0 mg/kg inorganic arsenic if that were the only toxin to which you were exposed. But when multiple toxins are present, as they most likely are in our diet, the cutoff for inorganic arsenic is set at 0.002 mg/kg. The FDA allows 38,000 times more inorganic arsenic than the EPA; that seems grievously negligent but in reality, the amount in commercial fish is significantly lower. It’s like saying you must be at least 2 inches tall, by the EPA’s standards, or 5 inches tall, by the FDA’s standards, to go on a rollercoaster ride.
One last note: the limit for methylmercury consumption is ~0.4 ug/kg/d, which is approximately 28 ug/d (for a 70 kg or 154 lb person). Even the most toxic salmon has methylmercury <100 ug/kg, meaning you can safely eat ~300 grams (10 ounces or about 3 servings) of salmon per day.
Round 2. Pesticides: Farmed vs. wild salmon
Global Assessment of Organic Contaminants in Farmed Salmon (Hites et al., 2004 Science)
These researcher went big-time, 700 fish! (appr. 1 ton of salmon)
Sources:
- Farmed Atlantic salmon: 8 major commercial suppliers.
- Wild Pacific salmon: chum, coho, chinook, pink, & sockeye from 3 different regions
- My personal favorite: Farmed Atlantic salmon fillets purchased by undercover secret agents in 16 cities in North America and Europe (Boston, Chicago, Denver, Edinburgh, Frankfurt, London, Los Angeles, New Orleans, New York, Oslo, Paris, San Francisco, Seattle, Toronto, Vancouver, and Washington DC.)
- They even analyzed samples of fish food covering over 80% of the global supply
Side note: even if your exact city or region isn’t on this list, I suspect the conclusions can be reasonably applied to just about everywhere.
Results:
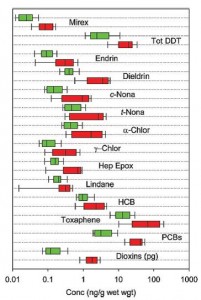
Figure 3. Contaminants present in Farmed (red) or wild (green) salmon. It looks like for every contaminant Farmed and wild are similar, but Farmed always has a little more (beware of the deceptive log scale)
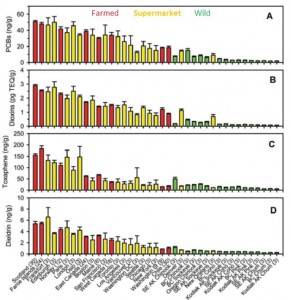
This figure is very busy. Concentration of contaminants in Farmed (red), supermarket Farmed Atlantic fillets (yellow), and wild (green) salmon. Focus on the cities listed at the bottom: the ones toward the left (Europe) are ultra-toxic; the ones on the right (Pacific [Alaska]) are the most safe. Conclusion from these data: Wild Pacific is safe, Farmed Atlantic is intermediate, and anything European is toxic. Avoid Scottish salmon like the plague. And microwave popcorn.
WRT farmed salmon, it looks like most of the problem is with the fish feed:
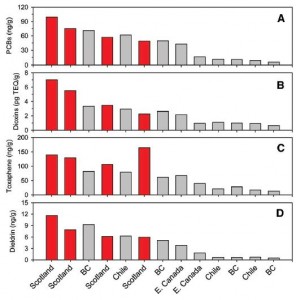
Figure 5. Contaminants in fish feed. European fish food is bunk (red bars). Pacific (BC British Columbia, Chile) and Atlantic (E. Canada) fish foods are OK (both in gray bars).
Conclusions:
WRT metals (Foran study): no difference between Farmed Atlantic and wild Pacific
WRT contaminants (Hites study): wild Pacific (Alaska and British Columbia, also Chilean) is good, supermarket Farmed Atlantic fillets are OK, and European is bad.
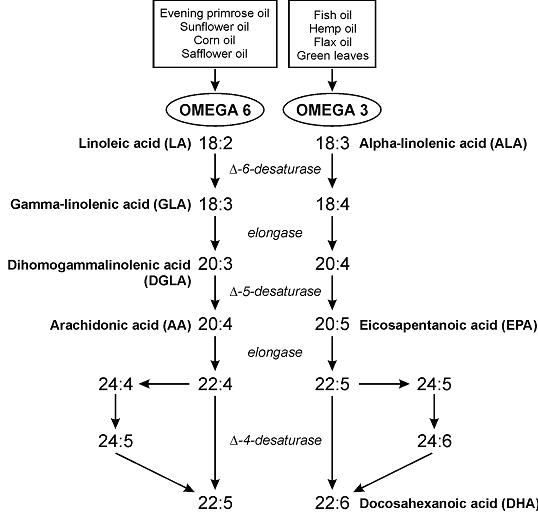
Round 3. Fatty acid composition as per www.NutritionData.com
Atlantic: Farmed vs. wild
Pacific coho: farmed vs. wild vs. silver Alaska native
Alaska: Silver native vs. King chinook
Total EPA + DHA: 1st place goes to farmed Atlantic: 1,966 mg EPA + DHA per 100 grams. On average, farmed salmon contains more EPA + DHA than wild salmon.
2nd place goes to silver Alaska native coho: 1,876 mg EPA + DHA
3rd place goes to wild Atlantic 1,436 mg EPA + DHA
Lowest were: wild Pacific coho (1,085 mg), Alaska King Chinook (1,150 mg), and wild Pacific Sockeye (1,172 mg). (all three are Pacific.)
-Farmed salmon has more EPA + DHA than wild salmon
-Atlantic salmon has more EPA + DHA than Pacific salmon
And there were even species-differences: Alaskan Silver native coho (1,876 mg) had much higher EPA + DHA than Alaskan King Chinook (1,150 mg).
EPA/DHA ratio: not entirely sure about the significance of this, but perhaps EPA is slightly better for physical health while DHA is slightly better for mental health (?) (future blog post topic?)
Average 0.6 (all salmon have slightly more EPA than DHA). Most EPA (highest EPA/DHA ratio): Farmed Atlantic & wild Pacific Sockeye (0.8). Most DHA (lowest EPA/DHA ratio): wild Atlantic (0.3) & Silver Alaska native coho (0.4)
Conclusions: WRT contaminants, wild Pacific seems best, farmed Atlantic is OK, and European is bad.
WRT EPA + DHA, Farmed Atlantic and silver Alaska native coho were best and wild Pacific was the lowest. IMHO the benefits of DHA & EPA outweigh the malefits of contaminants because the dose of EPA + DHA in a serving of salmon is sufficient to reap many of the benefits of EPA & DHA, while the dose of contaminants is too low to cause harm. Therefore I’m going to stick with Farmed Atlantic. OOTH if silver Alaska native coho is similar to Kodiak salmon (which I think it is), then it has the lowest contaminants as per the Hites study and 2nd highest EPA DHA as per nutritiondata.
Winner: wild Pacific Kodiak or silver Alaska native coho
2nd place: Farmed Atlantic
calories proper